Advancements in Biotechnology
Technological advancements in biotechnology are propelling the growth of the biologic therapy market. Innovations in genetic engineering, monoclonal antibody production, and cell therapy are enhancing the efficacy and safety of biologic treatments. The biologic therapy market is benefiting from these advancements, as they enable the development of more targeted therapies with fewer side effects. For instance, the introduction of CAR-T cell therapies has revolutionized the treatment landscape for certain cancers, demonstrating remarkable success rates. The US biotechnology sector is projected to grow at a CAGR of 10% through 2025, indicating a robust pipeline of new biologic therapies. This growth is likely to foster increased investment in research and development, further driving the biologic therapy market.
Increased Healthcare Expenditure
Rising healthcare expenditure in the US is a crucial driver of the biologic therapy market. As healthcare budgets expand, there is a greater allocation of resources towards innovative treatments, including biologics. The biologic therapy market is experiencing growth as payers and providers recognize the long-term cost-effectiveness of biologic therapies in managing chronic conditions. In 2025, US healthcare spending is projected to reach approximately $4.5 trillion, with a substantial portion directed towards advanced therapies. This increase in funding is likely to facilitate broader access to biologic treatments, thereby expanding the market. Furthermore, as more patients gain insurance coverage for biologic therapies, the demand is expected to rise, further propelling the biologic therapy market.
Growing Demand for Targeted Therapies
The shift towards personalized medicine is driving the demand for targeted therapies within the biologic therapy market. Patients and healthcare providers are increasingly seeking treatments that are tailored to individual genetic profiles and disease characteristics. This trend is particularly evident in oncology, where targeted biologic therapies have shown improved efficacy compared to traditional treatments. The biologic therapy market is responding to this demand by developing therapies that focus on specific biomarkers, enhancing treatment precision. As of 2025, it is estimated that targeted therapies will account for over 50% of the total biologic therapy market, reflecting a significant shift in treatment paradigms. This growing preference for personalized approaches is likely to shape the future of the biologic therapy market.
Rising Prevalence of Chronic Diseases
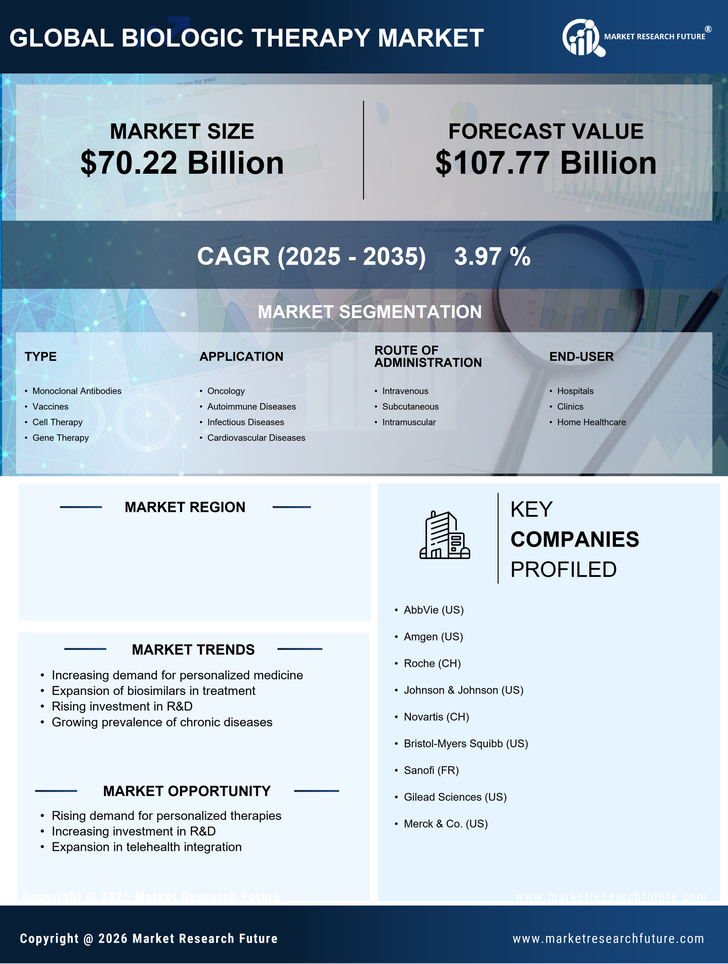
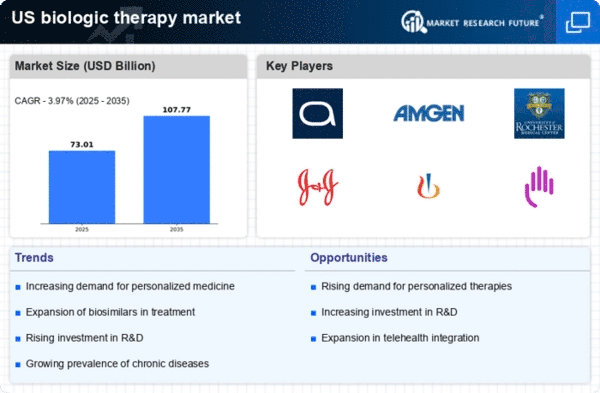
The increasing incidence of chronic diseases such as diabetes, cancer, and autoimmune disorders is a primary driver of the biologic therapy market. In the US, chronic diseases affect nearly 60% of adults, leading to a growing demand for effective treatment options. Biologic therapies, which target specific pathways in disease processes, are becoming essential in managing these conditions. The biologic therapy market is witnessing a surge in demand as healthcare providers seek innovative solutions to improve patient outcomes. This trend is expected to continue, with the market projected to reach approximately $300 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 8% from 2021 to 2026. As the population ages and the prevalence of chronic diseases rises, the biologic therapy market is likely to expand significantly.
Regulatory Support and Streamlined Approval Processes
Regulatory bodies in the US are increasingly supporting the biologic therapy market through streamlined approval processes. Initiatives such as the FDA's Breakthrough Therapy Designation aim to expedite the development and review of therapies that address unmet medical needs. This regulatory environment encourages pharmaceutical companies to invest in biologic therapies, as it reduces the time and cost associated with bringing new treatments to market. The biologic therapy market is likely to benefit from these favorable conditions, as more innovative therapies gain approval. In recent years, the FDA has approved a record number of biologics, with over 50 new products launched in 2020 alone. This trend is expected to continue, fostering a dynamic and competitive landscape in the biologic therapy market.