Rising Incidence of Chronic Diseases
The rising incidence of chronic diseases in the US is a significant driver for the biomarker technologies market. Conditions such as cancer, diabetes, and cardiovascular diseases are becoming increasingly prevalent, necessitating the development of effective diagnostic and therapeutic strategies. According to the CDC, chronic diseases account for approximately 70% of all deaths in the US, highlighting the urgent need for innovative solutions. Biomarkers are essential for early detection and monitoring of these diseases, which can lead to improved patient management and outcomes. As the burden of chronic diseases continues to grow, the demand for biomarker technologies is likely to expand correspondingly.
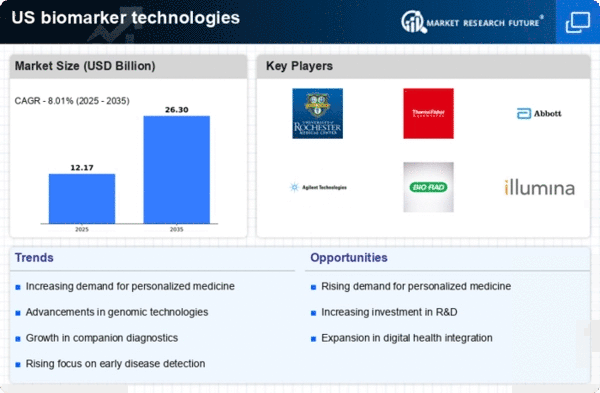
Growing Demand for Personalized Medicine
The increasing emphasis on personalized medicine is a pivotal driver for the biomarker technologies market. As healthcare shifts towards tailored treatments, biomarkers play a crucial role in identifying patient-specific therapies. This trend is evidenced by the projected growth of the personalized medicine market, which is expected to reach approximately $2 trillion by 2025. Biomarkers enable healthcare providers to predict treatment responses, thereby enhancing patient outcomes. The integration of biomarker technologies into clinical practice is likely to accelerate, as healthcare systems recognize the value of precision medicine. Consequently, the demand for innovative biomarker solutions is anticipated to rise, propelling the biomarker technologies market forward.
Regulatory Support for Biomarker Development
Regulatory support for biomarker development is emerging as a vital driver for the biomarker technologies market. Agencies such as the FDA are actively promoting the use of biomarkers in drug development and clinical trials. The establishment of guidelines for biomarker qualification has streamlined the approval process, encouraging pharmaceutical companies to invest in biomarker research. This regulatory environment fosters innovation and enhances the credibility of biomarker technologies. As regulatory frameworks continue to evolve, the biomarker technologies market is likely to benefit from increased collaboration between regulatory bodies and industry stakeholders, facilitating the introduction of new biomarker-based solutions.
Technological Advancements in Diagnostic Tools
Technological innovations in diagnostic tools are significantly influencing the biomarker technologies market. The development of advanced imaging techniques and high-throughput screening methods has enhanced the ability to identify and validate biomarkers. For instance, the market for diagnostic imaging is projected to grow at a CAGR of around 7% from 2023 to 2028. These advancements facilitate earlier disease detection and more accurate prognoses, which are essential for effective treatment planning. As diagnostic technologies continue to evolve, the biomarker technologies market is expected to benefit from increased adoption and integration of these sophisticated tools into clinical workflows.
Increased Investment in Research and Development
Investment in research and development (R&D) is a critical driver for the biomarker technologies market. Funding from both public and private sectors is essential for advancing biomarker discovery and validation. In recent years, federal funding for biomedical research has seen a notable increase, with the National Institutes of Health (NIH) budget reaching approximately $45 billion in 2025. This influx of capital supports innovative research initiatives aimed at identifying novel biomarkers for various diseases. As R&D efforts intensify, the biomarker technologies market is expected to experience significant growth, driven by the continuous emergence of new biomarker applications.
.png)