Increased Awareness and Education
Growing awareness regarding the benefits of bone growth stimulators is a crucial driver for the market. Educational initiatives by healthcare organizations and advocacy groups have led to a better understanding of bone health and the importance of timely intervention. This heightened awareness is particularly evident among healthcare professionals, who are increasingly recommending these devices for patients with bone healing challenges. In the US, the market is projected to grow by approximately 5% annually, as more patients seek out these solutions. Furthermore, the dissemination of information through digital platforms and community outreach programs has empowered patients to take proactive steps in managing their bone health. Consequently, the bone growth-stimulator market is likely to experience sustained growth as awareness continues to rise.
Supportive Reimbursement Policies
The presence of favorable reimbursement policies is a significant factor driving the bone growth-stimulator market. Insurance coverage for these devices has improved, making them more accessible to patients. In the US, many private insurers and government programs now include bone growth stimulators in their coverage plans, which alleviates the financial burden on patients. This trend is expected to continue, as policymakers recognize the cost-effectiveness of these devices in reducing long-term healthcare costs associated with untreated bone disorders. As reimbursement rates improve, the adoption of bone growth stimulators is likely to increase, further propelling market growth. The bone growth-stimulator market is anticipated to benefit from this supportive environment, leading to enhanced patient access and treatment outcomes.
Rising Incidence of Bone Disorders
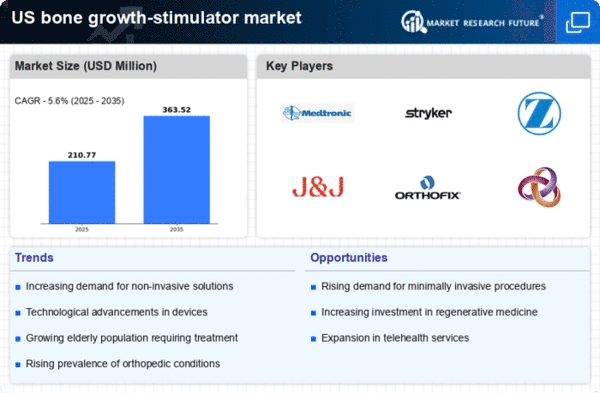
The increasing prevalence of bone disorders, such as osteoporosis and fractures, is a primary driver for the bone growth-stimulator market. In the US, it is estimated that osteoporosis affects approximately 10 million individuals, with an additional 44 million at risk. This growing patient population necessitates effective treatment options, thereby propelling demand for bone growth stimulators. Furthermore, the aging demographic contributes to this trend, as older adults are more susceptible to bone-related issues. The market is projected to witness a compound annual growth rate (CAGR) of around 6% over the next few years, driven by the need for innovative solutions to enhance bone healing and regeneration. As healthcare providers increasingly adopt these technologies, the bone growth-stimulator market is expected to expand significantly.
Technological Innovations in Treatment
Technological advancements in the field of bone growth stimulators are significantly influencing the market landscape. Innovations such as ultrasound and electrical stimulation devices have emerged, offering non-invasive options for patients. These technologies enhance the healing process by promoting cellular activity and improving blood flow to the affected areas. The US market has seen a surge in the adoption of these advanced devices, with a notable increase in sales, estimated to reach $1.5 billion by 2026. Additionally, the integration of smart technologies, such as wearable devices that monitor healing progress, is expected to further drive market growth. As healthcare providers seek to improve patient outcomes, the bone growth-stimulator market is likely to benefit from these cutting-edge developments.
Growing Demand for Minimally Invasive Procedures
The rising preference for minimally invasive procedures is shaping the bone growth-stimulator market. Patients increasingly favor treatments that involve less pain, shorter recovery times, and reduced hospital stays. Bone growth stimulators align well with this trend, as they offer non-invasive options for promoting bone healing. In the US, the demand for such procedures is on the rise, with a projected market growth rate of 7% over the next few years. This shift towards less invasive treatment modalities is likely to encourage healthcare providers to incorporate bone growth stimulators into their practice. As patient satisfaction becomes a priority, the bone growth-stimulator market is expected to thrive, driven by the desire for effective yet minimally invasive solutions.