Advancements in Material Science
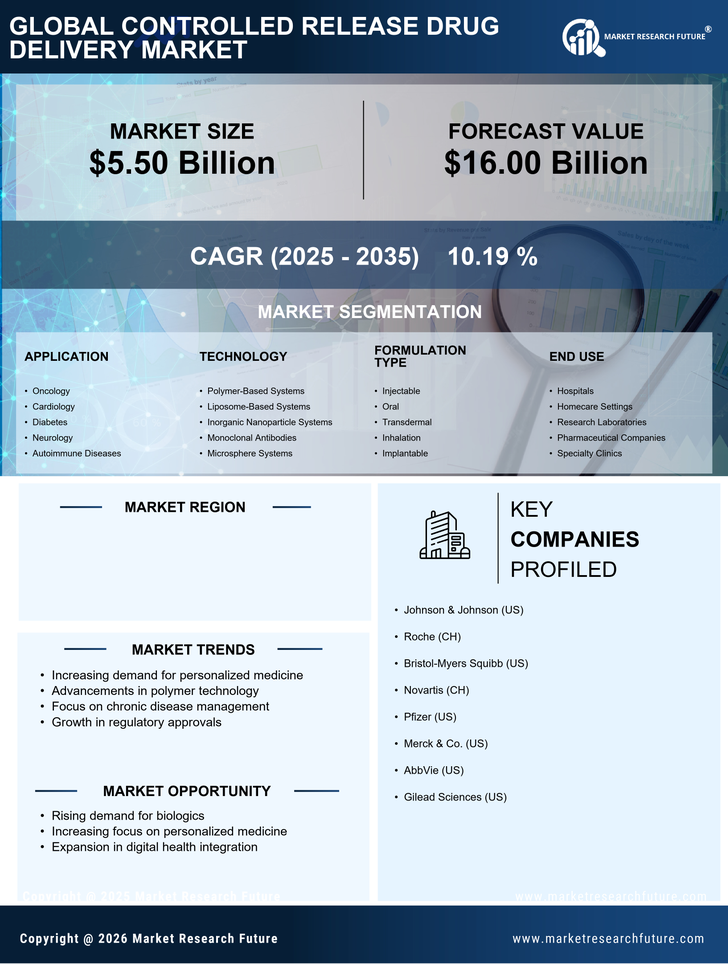
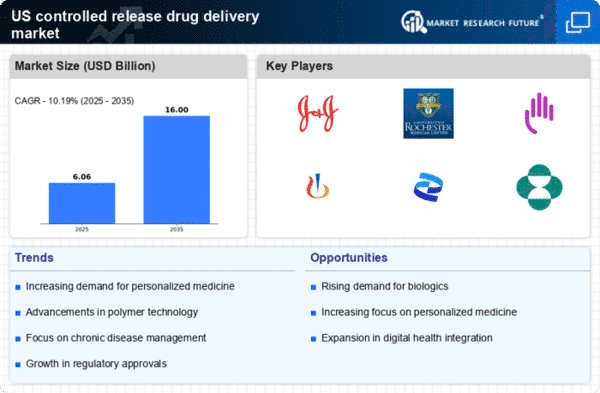
Innovations in material science are significantly impacting the controlled release-drug-delivery market. The development of biocompatible and biodegradable polymers has enabled the creation of more effective drug delivery systems. These materials allow for the precise control of drug release rates, enhancing the therapeutic efficacy of medications. Furthermore, the integration of nanotechnology into drug formulation is likely to improve the bioavailability of drugs, making them more effective at lower doses. As a result, the controlled release-drug-delivery market is poised for growth, with new products emerging that leverage these advancements to meet the needs of patients and healthcare providers.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is influencing the controlled release-drug-delivery market. Tailoring drug therapies to individual patient profiles can enhance treatment efficacy and minimize adverse effects. Controlled release systems are particularly well-suited for this approach, as they can be designed to deliver specific dosages based on a patient's unique needs. The increasing emphasis on personalized healthcare is likely to drive demand for advanced drug delivery systems that can accommodate varying patient requirements. As healthcare providers adopt more individualized treatment plans, the controlled release-drug-delivery market is expected to expand in response to this trend.
Rising Demand for Chronic Disease Management
The increasing prevalence of chronic diseases in the US is driving the growth of the controlled release drug delivery market. Conditions such as diabetes, hypertension, and cancer require long-term medication management, which controlled release systems can effectively provide. These systems enhance patient compliance by reducing the frequency of dosing, thereby improving therapeutic outcomes. According to recent estimates, chronic diseases account for approximately 70% of all deaths in the US, highlighting the urgent need for effective treatment options. The controlled release drug delivery market is expected to see a surge in demand as healthcare providers seek innovative solutions to manage these conditions more effectively.
Increased Investment in Research and Development
The controlled release-drug-delivery market is experiencing a surge in investment from both public and private sectors. This influx of funding is primarily directed towards research and development initiatives aimed at creating innovative drug delivery systems. In 2025, it is projected that R&D spending in the pharmaceutical sector will exceed $200 billion in the US, with a significant portion allocated to controlled release technologies. This investment is likely to accelerate the development of new products, enhancing the market's growth potential. As companies strive to bring novel solutions to market, the competitive landscape is expected to evolve, fostering further innovation.
Regulatory Support for Innovative Drug Delivery Systems
Regulatory bodies in the US are increasingly supportive of innovative drug delivery systems, which is beneficial for the controlled release-drug-delivery market. Streamlined approval processes and guidelines for new technologies are encouraging pharmaceutical companies to invest in the development of advanced drug delivery solutions. This regulatory environment fosters innovation and reduces time-to-market for new products. As a result, the controlled release-drug-delivery market is likely to see a rise in the introduction of novel therapies that leverage these supportive frameworks. The collaboration between industry and regulatory agencies is essential for ensuring that patients have access to the latest advancements in drug delivery.