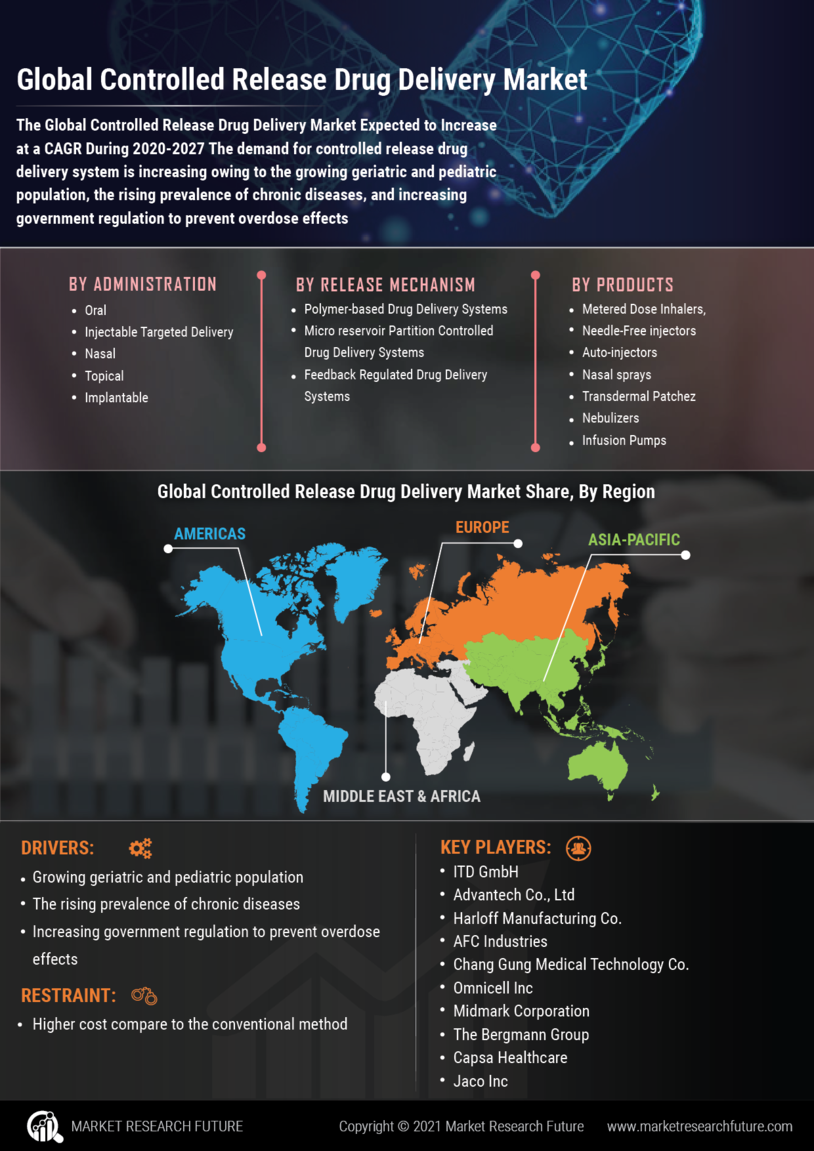
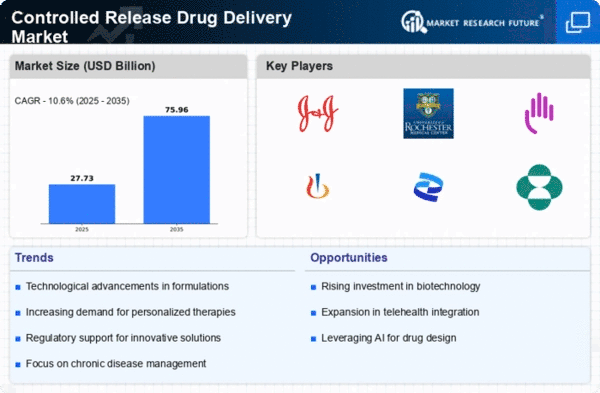
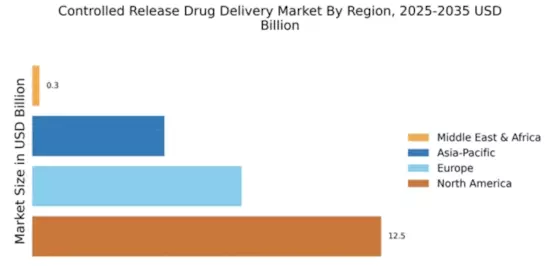
Market Growth Projections
The Global Controlled Release Drug Delivery Market Industry is poised for substantial growth, with projections indicating a market size of 25.1 USD Billion in 2024 and an anticipated increase to 76.0 USD Billion by 2035. This growth trajectory reflects a compound annual growth rate (CAGR) of 10.6% from 2025 to 2035. Such figures highlight the increasing adoption of controlled release technologies across various therapeutic areas, driven by advancements in formulation techniques and a growing emphasis on personalized medicine. The market's expansion is indicative of the broader trends in healthcare, where innovative drug delivery solutions are becoming integral to effective patient management.
Rising Demand for Targeted Therapies
The Global Controlled Release Drug Delivery Market Industry experiences an increasing demand for targeted therapies, which aim to enhance therapeutic efficacy while minimizing side effects. This trend is particularly evident in oncology, where controlled release systems allow for localized drug delivery, reducing systemic exposure. As a result, the market is projected to reach 25.1 USD Billion in 2024, reflecting a growing preference for precision medicine. The shift towards personalized treatment regimens is likely to drive innovation in controlled release technologies, enabling healthcare providers to tailor therapies to individual patient needs, thus improving overall treatment outcomes.
Increasing Prevalence of Chronic Diseases
The Global Controlled Release Drug Delivery Market Industry is significantly influenced by the rising prevalence of chronic diseases, including diabetes, cardiovascular disorders, and cancer. These conditions often require long-term medication regimens, making controlled release systems particularly advantageous. By providing sustained drug release, these systems improve patient adherence and therapeutic outcomes. As healthcare systems globally adapt to manage these chronic conditions more effectively, the demand for controlled release formulations is expected to surge. This trend suggests a robust market growth trajectory, aligning with the anticipated CAGR of 10.6% from 2025 to 2035.
Advancements in Drug Formulation Technologies
Technological advancements in drug formulation are pivotal in shaping the Global Controlled Release Drug Delivery Market Industry. Innovations such as nanotechnology and polymer-based systems facilitate the development of sophisticated drug delivery mechanisms. These advancements enable the creation of formulations that can release drugs at a controlled rate over extended periods, enhancing patient compliance and therapeutic effectiveness. As the industry evolves, it is anticipated that the market will expand significantly, with projections indicating a growth to 76.0 USD Billion by 2035. This growth underscores the importance of continuous research and development in the field of controlled release drug delivery.
Regulatory Support for Innovative Drug Delivery Systems
Regulatory bodies worldwide are increasingly supportive of innovative drug delivery systems, which is a crucial driver for the Global Controlled Release Drug Delivery Market Industry. Initiatives aimed at expediting the approval process for novel drug delivery technologies encourage pharmaceutical companies to invest in research and development. This regulatory environment fosters innovation, allowing for the introduction of advanced controlled release systems that can significantly enhance therapeutic efficacy. As a result, the market is likely to witness accelerated growth, driven by the introduction of new products that meet evolving patient and healthcare provider needs.
Growing Focus on Patient-Centric Drug Delivery Solutions
The Global Controlled Release Drug Delivery Market Industry is witnessing a paradigm shift towards patient-centric drug delivery solutions. This approach emphasizes the importance of enhancing patient experience and outcomes through tailored drug delivery systems. By focusing on factors such as ease of use, dosing frequency, and overall treatment satisfaction, pharmaceutical companies are developing controlled release formulations that align with patient preferences. This trend is expected to contribute to market growth, as patient-centric solutions are increasingly recognized as essential for improving adherence and treatment success rates in various therapeutic areas.