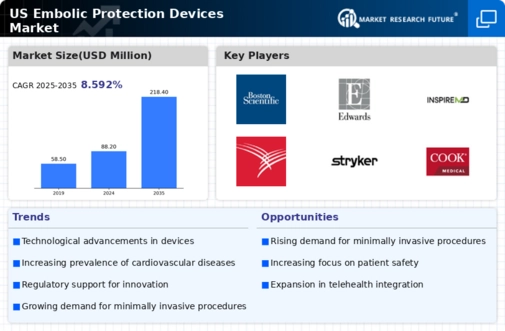
The US Embolic Protection Devices Market is characterized by significant competition and rapid technological advancements, driven by increased prevalence of cardiovascular diseases and a rising aging population. The market is populated by various players, including both established organizations and emerging companies, each vying for market share. Competition is primarily based on factors such as product quality, technological innovation, regulatory approvals, distribution channels, and pricing strategies.
As the market continues to evolve, companies are focusing on developing novel technologies and improving existing products to enhance patient outcomes while meeting the stringent requirements set forth by healthcare authorities. This competitive landscape is further influenced by growing investments in research and development, strategic partnerships, and collaborations aimed at expanding product portfolios and enhancing manufacturing capabilities.
Terumo Corporation has established a solid foothold in the US Embolic Protection Devices Market, thanks to its robust product line and commitment to innovation. The company is well-regarded for offering high-quality medical devices that address the critical needs of cardiovascular patients. Terumo Corporation boasts strengths in its research and development efforts, which bolster its ability to introduce advanced embolic protection solutions. Furthermore, the company's widespread distribution network across the US ensures product accessibility in hospitals and healthcare facilities.
With a strong emphasis on customer service and support, Terumo Corporation maintains a favorable reputation among healthcare professionals, which in turn fortifies its competitive position within this market segment.
Medtronic operates as a distinguished player in the US Embolic Protection Devices Market, leveraging its expansive experience and broad product portfolio to capture significant market share. The company is known for its sophisticated embolic protection devices that are integrated into a variety of cardiovascular procedures, ensuring optimal patient outcomes. Medtronic's strengths are further amplified by its ongoing commitment to research and development, allowing for the continuous introduction of innovative technologies that enhance the effectiveness of its offerings. Medtronic has also engaged in strategic mergers and acquisitions aimed at expanding its capabilities and market presence, ensuring a competitive edge.
Their dominant position in the embolic protection landscape is complemented by strong relationships with healthcare providers, rigorous regulatory compliance, and a reputation for quality management, making Medtronic a formidable competitor in the US market.