Market Analysis
In-depth Analysis of Viral Vector Manufacturing Market Industry Landscape
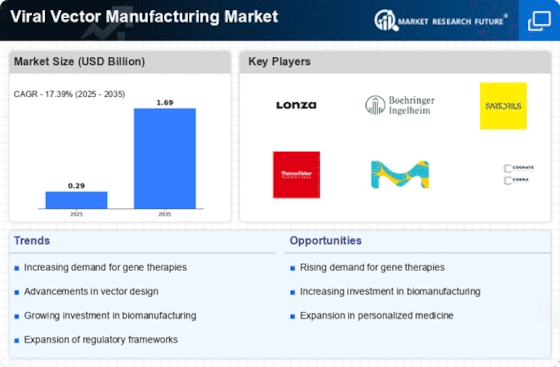
Growth of viral vector manufacturing has been driven by increased utilization of gene therapies, vaccines & cell-based medicines. Advanced therapeutic approaches rely on viral vectors to deliver genetic materials into target cells. The need for efficient and viable production processes for gene based therapeutics has contributed to driving demand in the Viral Vector Manufacturing Market.
Market dynamics are driven by Gene therapies and viral vector-based vaccinations. Gene therapy has proved effective in addressing genetic disorders and other diseases using AAVs or lentiviruses. Vaccine development relies on viral vectors especially for emerging infectious diseases (EIDs). There has been a high demand for rapid synthesis of large scale viral vectors for vaccine production during COVID-19 pandemic.
The market has been shaped by technology in the production of viral vectors. Adherent cell culture methods used in viral vector generation have either been replaced or supplemented with suspension cell culture systems, bioreactors or continuous manufacture methods. These advancements address issues related to scalability, process optimization and increased yields thereby enabling manufacturers meet global demand for these products.
Cutting-edge technologies involved in production of viral vectors keep academic institutions, CMOs, biopharmaceutical companies competitive amongst them-selves. In order to encourage innovation regarding robust yet low-cost manufacturing platforms as well as optimizing both upstream & downstream processes while achieving higher efficiency during production of infectious vials, there is need to maintain stiff competition within the sector among key players. Producers are also required to adapt their manufacturing techniques according to specific medical uses through joint efforts with developers of treatments using viral vectors.
Over the years, the need for viral vector production has helped shape ideas around gene and cell-based therapeutics globally in revolutionary medicines as well as public health emergencies. Rapid development of viral vectors for COVID-19 vaccines demonstrated that manufacturability is vital in global healthcare. This can be seen in the flexibility and adaptability of viral vector manufacturing processes to speed up vaccine research and production amidst pandemics.
The market dynamics of viral vector manufacturing are affected by regulatory factors due to the unique challenges and risks associated with gene therapy. Strict FDA standards, quality, safety, consistency should apply for American Therapeutic Vector Treatment products. Viral vector manufacturers need to adhere to these regulations to ensure they are accepted in the market.
Viral vector manufacturing technology adoption is influenced by cost. The inputs used, facility infrastructure as well as human labor affect costs incurred while producing viral vectors. Biopharmaceutical inventors must consider the economic viability of viral vector manufacturing based on therapeutic utilities, batch sizes use and customer needs. Gene therapies and vaccines require cost optimization and scalability for commercial success.















Leave a Comment