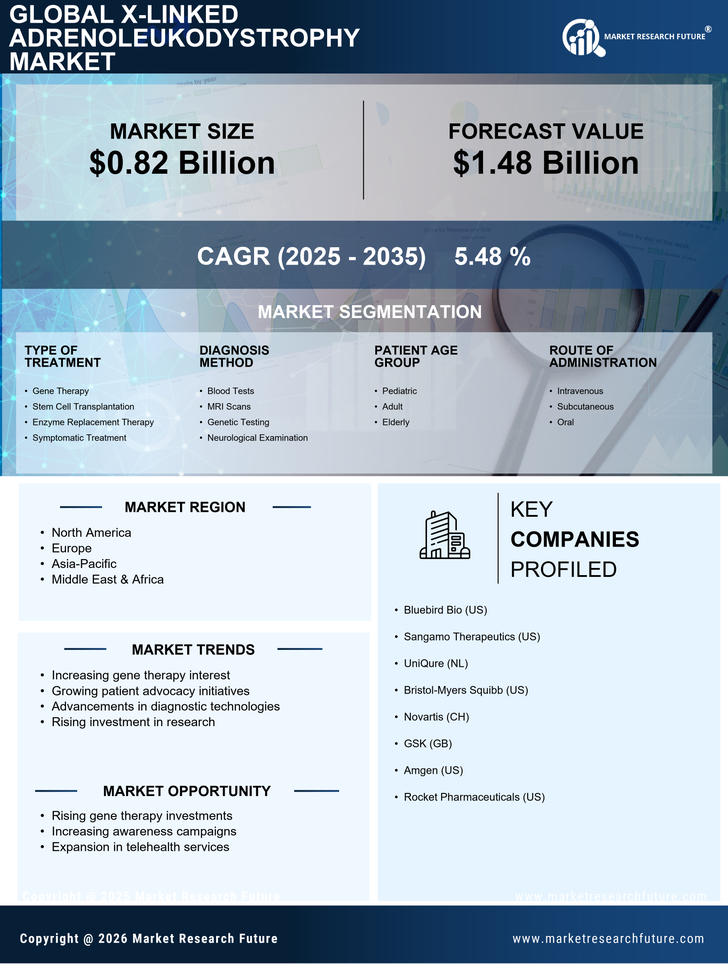
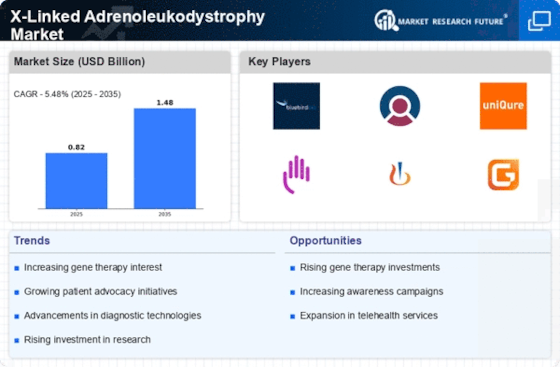
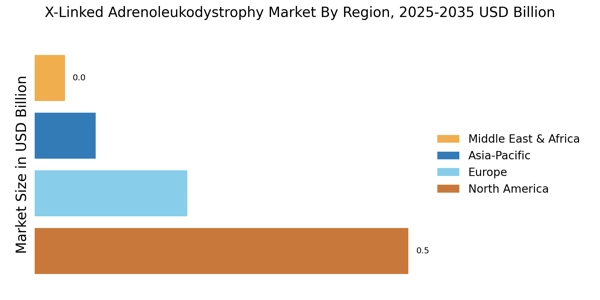
Advancements in Gene Therapy
Recent advancements in gene therapy represent a transformative force within the X-Linked Adrenoleukodystrophy Market. Innovative approaches, such as the use of viral vectors to deliver corrective genes, have shown promise in clinical trials. These therapies aim to address the underlying genetic defect responsible for X-ALD, potentially offering a long-term solution for affected individuals. The market is witnessing a surge in investment directed towards research and development of these novel therapies. As more gene therapies receive regulatory approval, the landscape of treatment options for X-ALD is likely to expand, attracting both patients and healthcare providers. This evolution in treatment modalities could significantly alter the trajectory of the X-Linked Adrenoleukodystrophy Market.
Collaboration Between Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, research institutions, and patient advocacy groups, is emerging as a key driver for the X-Linked Adrenoleukodystrophy Market. These partnerships are fostering a multidisciplinary approach to research and development, which is essential for addressing the complexities of X-ALD. By pooling resources and expertise, stakeholders can accelerate the discovery and commercialization of new therapies. Additionally, collaborative efforts often lead to shared clinical trials, which can reduce costs and improve the efficiency of the drug development process. This synergy is likely to enhance the overall landscape of the X-Linked Adrenoleukodystrophy Market, ultimately benefiting patients and healthcare providers alike.
Increasing Awareness and Diagnosis
The rising awareness surrounding X-Linked Adrenoleukodystrophy (X-ALD) is a pivotal driver for the X-Linked Adrenoleukodystrophy Market. Enhanced educational initiatives and advocacy from patient organizations have led to improved diagnostic rates. As healthcare professionals become more informed about the disease, the number of diagnosed cases is likely to increase. This trend is supported by data indicating that early diagnosis can significantly improve patient outcomes. Consequently, the demand for diagnostic tools and therapies is expected to rise, thereby propelling the market forward. Furthermore, the integration of genetic testing into routine screenings may facilitate earlier identification of at-risk individuals, further expanding the market potential for X-ALD treatments.
Growing Investment in Rare Disease Research
The increasing investment in research focused on rare diseases, including X-Linked Adrenoleukodystrophy, is a crucial driver for the X-Linked Adrenoleukodystrophy Market. Governments and private entities are recognizing the need for targeted therapies for conditions that have historically been overlooked. This influx of funding is facilitating the development of new treatment options and enhancing the understanding of X-ALD. According to recent reports, funding for rare disease research has seen a substantial rise, which is likely to accelerate the pace of innovation in the X-ALD space. As a result, the market is expected to benefit from a broader array of therapeutic options and improved patient care.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development of innovative therapies for X-Linked Adrenoleukodystrophy, which is a significant driver for the X-Linked Adrenoleukodystrophy Market. Initiatives such as orphan drug designations and expedited review processes are designed to encourage the development of treatments for rare diseases. This regulatory environment is fostering a climate of innovation, allowing companies to bring new therapies to market more efficiently. As a result, the number of available treatment options for X-ALD is likely to increase, which could enhance patient access to necessary therapies. The supportive regulatory framework is expected to play a vital role in shaping the future of the X-Linked Adrenoleukodystrophy Market.