-
REPORT PROLOGUE
-
MARKET INTRODUCTION
-
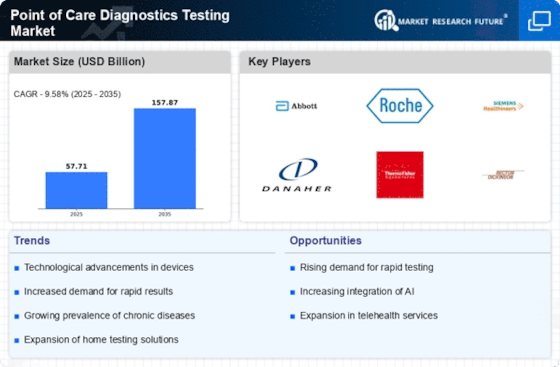
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
-
Forecasting Cellulite Type
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Value Chain Analysis
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Impact on Supply Chain
- Regional Impact
- Opportunity and Threat Analysis
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY PRODUCT
-
Overview
-
Glucose Monitoring
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Strips
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Meters
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Lancets
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Cardiometabolic Monitoring
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Cardiac Marker Testing Products
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Blood Gas/Electrolyte Testing Products
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
HbA1c Testing Products
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Infectious Disease Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
HIV Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Respiratory Infection Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Hepatitis Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Sexually Transmitted Disease Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Others
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Coagulation Monitoring
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
PT/INR Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
ACT/APTT Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Pregnancy & Fertility Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Hematology Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Cancer/Tumor Marker Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Urinalysis Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Cholesterol
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Drugs-of-Abuse Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Others
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY END USER
-
Overview
-
Hospitals & Clinics
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Diagnostic Centers
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Homecare/Self Testing
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Others
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE
-
Overview
-
OTC Testing Products
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Prescription-based testing products
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY PLATFORM
-
Overview
-
Immunoassays
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Dipsticks
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Molecular Diagnostics
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
Others
-
Market Estimates & Forecast, by Region, 2022–2030
-
Market Estimates & Forecast, by Country, 2022–2030
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY REGION
-
Overview
-
North America
- US
- Canada
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
-
Asia-Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia-Pacific
-
Rest of the World
- Middle East
- Africa
- Latin America
-
COMPANY LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market Share Analysis
-
Major Growth Strategy in the Global Point of Care Diagnostics Testing Market
-
Competitive Benchmarking
-
Leading Players in Terms of Number of Developments in the Global Point of Care Diagnostics Testing Market
-
Key developments and Growth Strategies
- New ProductLaunch/Service Deployment
- Merger &Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales & Operating Income, 2020
- Major Players R&D Expenditure, 2020
-
COMPANY PROFILES
-
Abbott Laboratories
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Siemens Healthineers GmbH
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Chembio Diagnostics, Inc.
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
F. Hoffmann-La Roche Ltd
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Johnson & Johnson Services, Inc.
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Becton, Dickinson and Company
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Danaher Corporation
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
PTS Diagnostics
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Nova Biomedical
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Quidel Corporation
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Sekisui Diagnostics
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Trinity Biotech
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
AccuBioTech Co., Ltd.
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Instrumentation Laboratory Company (Werfen)
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
EKF Diagnostics Holdings plc
- Company Overview
- Product Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
LIST OF TABLES
-
GLOBAL Point of Care Diagnostics Testing MARKET SYNOPSIS, 2022–2030
-
GLOBAL Point of Care Diagnostics Testing MARKET ESTIMATES &FORECAST, 2022–2030(USD MILLION)
-
GLOBAL Point of Care Diagnostics Testing MARKET, BYPRODUCT, 2022–2030 (USD MILLION)
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY END USER,2022–2030 (USD MILLION)
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
GLOBAL Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
NORTH AMERICA OF CARE DIAGNOSTICS/TESTING MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET, BY END USER,2022–2030 (USD MILLION)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
US: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
US Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
US: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
US: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
CANADA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
CANADA Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
CANADA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
CANADA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
EUROPE: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
EUROPE: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
EUROPE: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
EUROPE: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
GERMANY: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
GERMANY: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
GERMANY: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
GERMANY: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
FRANCE: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
FRANCE: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
FRANCE: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
FRANCE: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
UK: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
UK: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
UK: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
UK: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
ITALY: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
ITALY: Point of Care Diagnostics Testing MARKET, BY END USER2022–2030 (USD MILLION)
-
ITALY: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
ITALY: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
SPAIN: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
SPAIN: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
SPAIN: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
SPAIN: Point of Care Diagnostics Testing MARKET, BYPLATFORM2022–2030 (USD MILLION)
-
REST OF EUROPE: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
REST OF EUROPE: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
REST OF EUROPE: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
REST OF EUROPE: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
CHINA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
CHINA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
CHINA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
CHINA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
INDIA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
INDIA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
INDIA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
INDIA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
JAPAN: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
JAPAN: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
JAPAN: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
JAPAN: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
SOUTH KOREA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
SOUTH KOREA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
SOUTH KOREA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
SOUTH KOREA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
AUSTRALIA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
AUSTRALIA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
AUSTRALIA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
AUSTRALIA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
REST OF ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
REST OF ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
REST OF ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
REST OF ASIA-PACIFIC: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
REST OF WORLD: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
REST OF WORLD: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
REST OF WORLD: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
REST OF WORLD: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
MIDDLE EAST: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
MIDDLE EAST: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
MIDDLE EAST: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
MIDDLE EAST: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
AFRICA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
AFRICA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
AFRICA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
AFRICA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
LATIN AMERICA: Point of Care Diagnostics Testing MARKET, BY PRODUCT, 2022–2030 (USD MILLION)
-
LATIN AMERICA: Point of Care Diagnostics Testing MARKET, BY END USER, 2022–2030 (USD MILLION)
-
LATIN AMERICA: Point of Care Diagnostics Testing MARKET, BY MODE OF PURCHASE, 2022–2030 (USD MILLION)
-
LATIN AMERICA: Point of Care Diagnostics Testing MARKET, BY PLATFORM, 2022–2030 (USD MILLION)
-
LIST OF FIGURES
-
RESEARCH PROCESS
-
MARKET STRUCTURE FOR THE GLOBAL POINT OF CAREDIAGNOSTICS/TESTING MARKET
-
MARKET DYNAMICS FOR THE GLOBAL Point of Care Diagnostics Testing MARKET
-
GLOBAL Point of Care Diagnostics Testing MARKET SHARE, BY PRODUCT, 2020 (%)
-
GLOBAL Point of Care Diagnostics Testing MARKET SHARE, BY END USER, 2020 (%)
-
GLOBAL Point of Care Diagnostics Testing MARKET SHARE, BY MODE OF PURCHASE, 2020 (%)
-
GLOBAL Point of Care Diagnostics Testing MARKET SHARE, BY PLATFORM, 2020 (%)
-
GLOBAL Point of Care Diagnostics Testing MARKET SHARE, BY REGION, 2020 (%)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET SHARE, BY PRODUCT, 2020 (%)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET SHARE, BY END USER, 2020 (%)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET SHARE, BY MODE OF PURCHASE, 2020 (%)
-
NORTH AMERICAPoint of Care Diagnostics Testing MARKET SHARE, BY PLATFORM, 2020 (%)
-
EUROPEPoint of Care Diagnostics Testing MARKET SHARE, BY PRODUCT, 2020 (%)
-
EUROPEPoint of Care Diagnostics Testing MARKET SHARE, BY END USER, 2020 (%)
-
EUROPEPoint of Care Diagnostics Testing MARKET SHARE, BY MODE OF PURCHASE, 2020 (%)
-
EUROPEPoint of Care Diagnostics Testing MARKET SHARE, BY PLATFORM, 2020 (%)
-
ASIA-PACIFICPoint of Care Diagnostics Testing MARKET SHARE, BY PRODUCT, 2020 (%)
-
ASIA-PACIFICPoint of Care Diagnostics Testing MARKET SHARE, BY END USER, 2020 (%)
-
ASIA-PACIFICPoint of Care Diagnostics Testing MARKET SHARE, BY MODE OF PURCHASE, 2020 (%)
-
ASIA-PACIFICPoint of Care Diagnostics Testing MARKET SHARE, BY PLATFORM, 2020 (%)
-
REST OF THE WORLDPoint of Care Diagnostics Testing MARKET SHARE, BY PRODUCT, 2020 (%)
-
REST OF THE WORLD Point of Care Diagnostics Testing MARKET SHARE, BY END USER, 2020 (%)
-
REST OF THE WORLDPoint of Care Diagnostics Testing MARKET SHARE, BY MODE OF PURCHASE, 2020 (%)
-
REST OF THE WORLD Point of Care Diagnostics Testing MARKET SHARE, BY PLATFORM, 2020 (%)
-
ABBOTT LABORATORIES .: KEY FINANCIALS
-
ABBOTT LABORATORIES : SEGMENTAL REVENUE
-
ABBOTT LABORATORIES : REGIONAL REVENUE
-
SIEMENS HEALTHINEERS GMBH : KEY FINANCIALS
-
SIEMENS HEALTHINEERS GMBH : SEGMENTAL REVENUE
-
SIEMENS HEALTHINEERS GMBH : REGIONAL REVENUE
-
CHEMBIO DIAGNOSTICS, INC. : KEY FINANCIALS
-
CHEMBIO DIAGNOSTICS, INC. : SEGMENTAL REVENUE
-
CHEMBIO DIAGNOSTICS, INC. : REGIONAL REVENUE
-
F. HOFFMANN-LA ROCHE LTD.: KEY FINANCIALS
-
F. HOFFMANN-LA ROCHE LTD.: SEGMENTAL REVENUE
-
F. HOFFMANN-LA ROCHE LTD.: REGIONAL REVENUE
-
JOHNSON & JOHNSON SERVICES, INC. : KEY FINANCIALS
-
JOHNSON & JOHNSON SERVICES, INC. : SEGMENTAL REVENUE
-
JOHNSON & JOHNSON SERVICES, INC. : REGIONAL REVENUE
-
BECTON, DICKINSON AND COMPANY : KEY FINANCIALS
-
BECTON, DICKINSON AND COMPANY : SEGMENTAL REVENUE
-
BECTON, DICKINSON AND COMPANY : REGIONAL REVENUE
-
DANAHER CORPORATION : KEY FINANCIALS
-
DANAHER CORPORATION : SEGMENTAL REVENUE
-
DANAHER CORPORATION : REGIONAL REVENUE
-
PTS DIAGNOSTICS: KEY FINANCIALS
-
PTS DIAGNOSTICS: SEGMENTAL REVENUE
-
PTS DIAGNOSTICS: REGIONAL REVENUE
-
NOVA BIOMEDICAL : KEY FINANCIALS
-
NOVA BIOMEDICAL : SEGMENTAL REVENUE
-
NOVA BIOMEDICAL : REGIONAL REVENUE
-
QUIDEL CORPORATION : KEY FINANCIALS
-
QUIDEL CORPORATION : SEGMENTAL REVENUE
-
QUIDEL CORPORATION : REGIONAL REVENUE
-
SEKISUI DIAGNOSTICS : KEY FINANCIALS
-
SEKISUI DIAGNOSTICS : SEGMENTAL REVENUE
-
SEKISUI DIAGNOSTICS : REGIONAL REVENUE
-
TRINITY BIOTECH : KEY FINANCIALS
-
TRINITY BIOTECH : SEGMENTAL REVENUE
-
TRINITY BIOTECH : REGIONAL REVENUE
-
ACCUBIOTECH CO., LTD.: KEY FINANCIALS
-
ACCUBIOTECH CO., LTD.: SEGMENTAL REVENUE
-
ACCUBIOTECH CO., LTD.: REGIONAL REVENUE
-
INSTRUMENTATION LABORATORY COMPANY (WERFEN): KEY FINANCIALS
-
INSTRUMENTATION LABORATORY COMPANY (WERFEN): SEGMENTAL REVENUE
-
INSTRUMENTATION LABORATORY COMPANY (WERFEN): REGIONAL REVENUE
-
EKF DIAGNOSTICS HOLDINGS PLC: KEY FINANCIALS
-
EKF DIAGNOSTICS HOLDINGS PLC: SEGMENTAL REVENUE
-
EKF DIAGNOSTICS HOLDINGS PLC: REGIONAL REVENUE
-
"








Leave a Comment