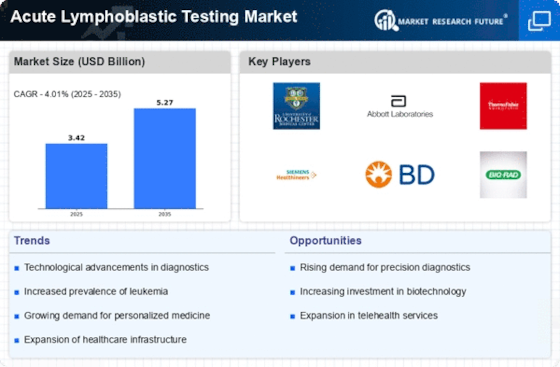
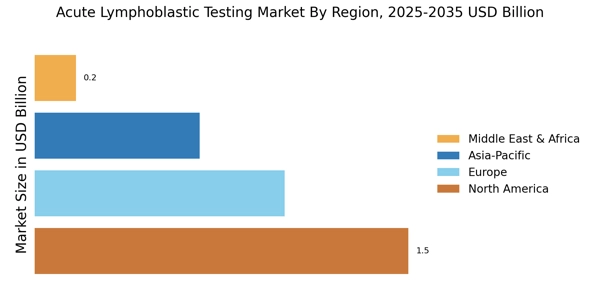
Increased Funding for Cancer Research
The surge in funding for cancer research is a notable driver of the Acute Lymphoblastic Testing Market. Governments and private organizations are allocating substantial resources to enhance research initiatives aimed at understanding and treating ALL. This influx of funding facilitates the development of novel diagnostic tools and methodologies, which are essential for improving patient care. For instance, recent reports indicate that funding for cancer research has increased by over 15% in the last few years, reflecting a growing commitment to combatting this disease. As research progresses, the introduction of new testing protocols and technologies is anticipated, further stimulating the Acute Lymphoblastic Testing Market. The collaboration between research institutions and diagnostic companies is likely to yield innovative solutions that address the evolving needs of healthcare providers and patients alike.
Growing Awareness and Education Programs
The rise in awareness and education programs regarding acute lymphoblastic leukemia is significantly impacting the Acute Lymphoblastic Testing Market. Initiatives aimed at educating healthcare professionals and the general public about the symptoms and importance of early detection are becoming increasingly prevalent. These programs are instrumental in promoting timely testing and diagnosis, which is crucial for improving patient outcomes. As awareness levels rise, more individuals are likely to seek testing, thereby driving demand within the market. Additionally, partnerships between healthcare organizations and advocacy groups are fostering a culture of proactive health management. This trend is expected to enhance the visibility of acute lymphoblastic testing options, ultimately contributing to the growth of the Acute Lymphoblastic Testing Market.
Regulatory Support for Diagnostic Testing
Regulatory bodies are playing a pivotal role in shaping the Acute Lymphoblastic Testing Market through the establishment of supportive frameworks for diagnostic testing. Recent regulatory updates have streamlined the approval processes for new testing technologies, encouraging innovation and market entry. This regulatory support is crucial for ensuring that high-quality testing solutions are accessible to healthcare providers and patients. As a result, the market is witnessing an influx of new products designed to meet stringent regulatory standards. Furthermore, the emphasis on evidence-based practices is likely to drive the adoption of advanced testing methodologies, as healthcare providers seek to comply with regulatory requirements. This dynamic environment fosters competition among diagnostic companies, ultimately benefiting the Acute Lymphoblastic Testing Market.
Technological Innovations in Testing Methods
Technological advancements in diagnostic testing methods are significantly influencing the Acute Lymphoblastic Testing Market. Innovations such as next-generation sequencing (NGS) and polymerase chain reaction (PCR) techniques have revolutionized the way ALL is diagnosed. These technologies offer enhanced sensitivity and specificity, allowing for the detection of minimal residual disease, which is crucial for patient management. The market for molecular diagnostics is projected to grow substantially, with estimates suggesting a compound annual growth rate of over 10% in the coming years. As laboratories increasingly adopt these cutting-edge technologies, the demand for acute lymphoblastic testing is expected to rise correspondingly. This trend not only improves patient outcomes but also drives competition among diagnostic companies to develop more efficient and reliable testing solutions.
Rising Incidence of Acute Lymphoblastic Leukemia
The increasing prevalence of acute lymphoblastic leukemia (ALL) is a primary driver for the Acute Lymphoblastic Testing Market. Recent statistics indicate that ALL accounts for approximately 25% of all childhood cancers, necessitating effective diagnostic testing. As the incidence rates rise, healthcare providers are compelled to adopt advanced testing methodologies to ensure timely and accurate diagnosis. This trend is likely to bolster the demand for innovative testing solutions, thereby propelling market growth. Furthermore, the heightened awareness among healthcare professionals and patients regarding early detection and treatment options is expected to contribute to the expansion of the Acute Lymphoblastic Testing Market. The need for precise testing is underscored by the complexity of ALL, which requires tailored therapeutic approaches based on individual patient profiles.