China : Robust Growth Driven by Innovation
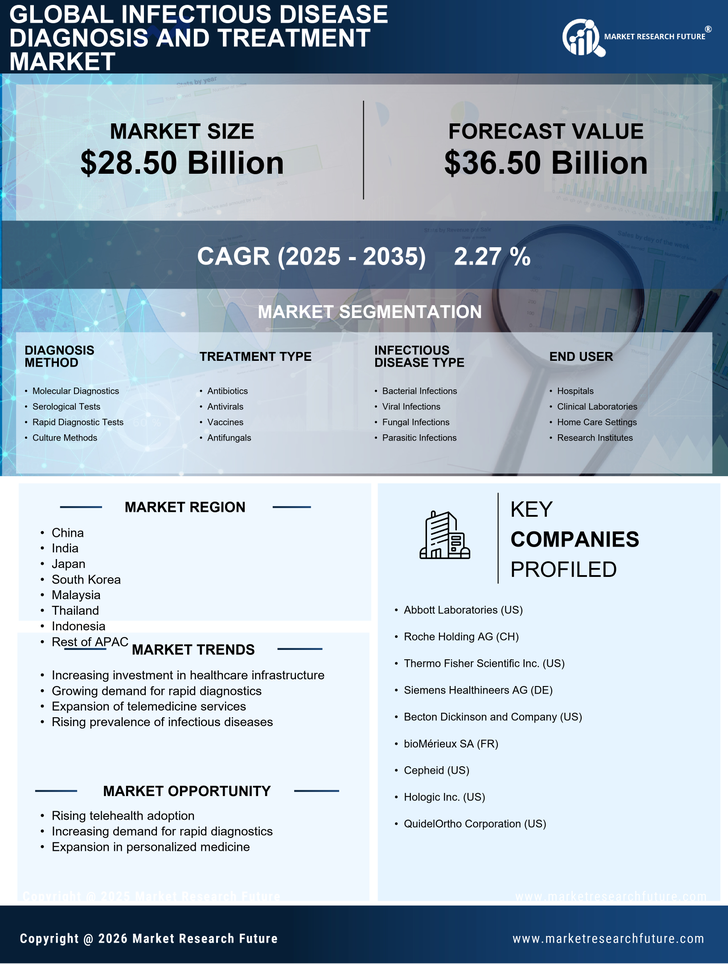
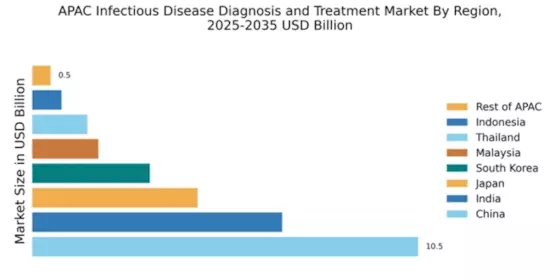
China holds a commanding 10.5% market share in the infectious disease diagnosis and treatment sector, valued at approximately $XX billion. Key growth drivers include increasing healthcare expenditure, rapid urbanization, and a rising prevalence of infectious diseases. Government initiatives, such as the Healthy China 2030 plan, aim to enhance healthcare infrastructure and accessibility. The regulatory environment is becoming more favorable, with streamlined approval processes for diagnostic products.
India : Rapid Growth in Healthcare Investments
India's market share stands at 6.8%, reflecting a burgeoning demand for infectious disease diagnostics. The growth is fueled by increasing awareness of healthcare, government initiatives like Ayushman Bharat, and a growing middle class. The demand for rapid testing and point-of-care diagnostics is on the rise, supported by advancements in technology and infrastructure improvements in urban areas.
Japan : Innovation and Quality Drive Market
Japan accounts for 4.5% of the APAC market, characterized by high-quality standards and advanced technology in diagnostics. The aging population and increasing healthcare needs are significant growth drivers. Regulatory frameworks are stringent, ensuring high safety and efficacy standards. The government supports research and development, fostering innovation in diagnostic technologies.
South Korea : Healthcare Innovation and Accessibility
South Korea holds a 3.2% market share, driven by a robust healthcare system and technological advancements. The government promotes health initiatives and invests in healthcare infrastructure, enhancing access to diagnostic services. The demand for rapid and accurate testing is growing, particularly in urban centers like Seoul and Busan, where healthcare facilities are concentrated.
Malaysia : Investment in Healthcare Infrastructure
Malaysia's market share is 1.8%, with significant growth potential driven by government initiatives like the National Health Policy. The increasing prevalence of infectious diseases and a focus on improving healthcare access are key growth factors. The regulatory environment is evolving, supporting the introduction of innovative diagnostic solutions in the market.
Thailand : Healthcare Reforms and Investments
Thailand's market share is 1.5%, supported by ongoing healthcare reforms and investments in public health. The demand for infectious disease diagnostics is rising, driven by increased awareness and government initiatives. Key cities like Bangkok and Chiang Mai are central to market activities, with a competitive landscape featuring both local and international players.
Indonesia : Focus on Healthcare Accessibility
Indonesia's market share is 0.8%, with significant opportunities for growth in infectious disease diagnostics. The government is focusing on improving healthcare access through initiatives like the National Health Insurance program. Urban areas such as Jakarta are seeing increased demand for diagnostic services, although challenges remain in rural healthcare delivery.
Rest of APAC : Varied Growth Across Sub-regions
The Rest of APAC holds a 0.5% market share, characterized by diverse healthcare landscapes and varying levels of infrastructure development. Growth is driven by localized healthcare needs and government initiatives aimed at improving public health. Each country presents unique challenges and opportunities, influenced by economic conditions and regulatory environments.