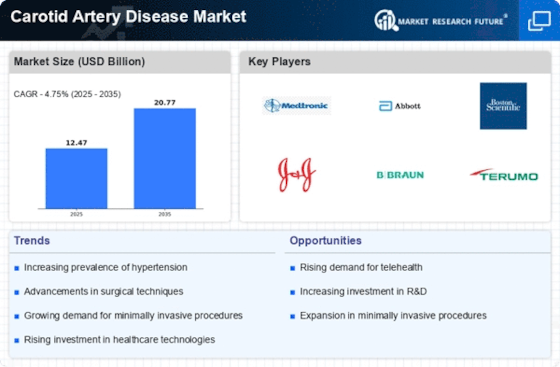
Top Industry Leaders in the Carotid Artery Disease Market
 Latest Carotid Artery Disease Treatment Companies Update
Latest Carotid Artery Disease Treatment Companies Update
-
August 2022: Following recent CE Mark approval, Medtronic announced the introduction of its newest drug-eluting coronary stent, the Onyx Frontier drug-eluting stent (DES). The Onyx Frontier drug-eluting stent (DES) presents a novel delivery system that extends the capabilities and clinical insights established with the Resolute Onyx DES. Utilizing the identical best-in-class stent platform as Resolute Onyx DES, the DES augments acute efficacy and deliverability in the most difficult cases through an improved delivery system. Significant design modifications of the Onyx Frontier DES, such as an innovative dual-layer balloon, a reduced crossing profile, and enhanced catheter flexibility, resulted in a 16% enhancement in deliverability when compared to the Resolute Onyx DES of the previous iteration. Moreover, radial strength was not compromised. Resolute Onyx's clinical data and indications are carried over to Onyx Frontier, including approval for left main PCI, bifurcation lesions, and one month of dual antiplatelet therapy (DAPT) in patients at high risk of hemorrhage.
-
May 2023: A prominent provider from India, Dr. Reddy's Laboratories Ltd., has introduced Regadenoson Injection to the U.S. market. The generic therapeutic equivalent of Lexiscan (Regadenoson) injection, which has received approval from the U.S. Food and Drug Administration (USFDA), is available. As per the press release issued by the organization, Dr. Reddy's Regadenoson Injection is available in pre-filled single-dose syringes containing 0.4 mg/5 mL (0.08 mg/mL). The purpose of this injection is to diagnose coronary artery disease in the heart. It is administered to patients who are unable to exercise for their stress test, per health professionals. As per the Mayo Clinics, this medication aids in the detection of coronary artery disease by dilatation of the coronary arteries and elevation of blood flow.
List of Carotid Artery Disease Treatment Key companies in the market
- Medtronic Plc
- Pfizer
- GE Healthcare
- Cardinal Health
- Eli Lily and Company
- AdvanceCor GmbH
- Teleflex, Siemens Healthcare
- Daiichi Sankyo Co. Ltd.
- Abbot Laboratories
- Bristol-Myers Squibb
- Merck & Cp. Inc.
- Silk Road Medical
- Stryker Corporation