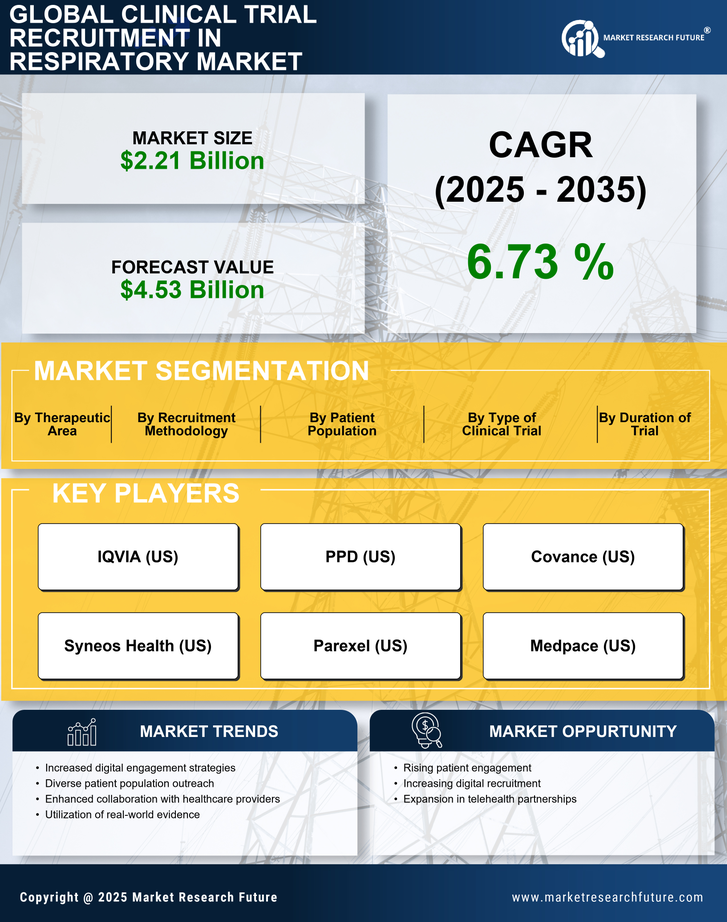
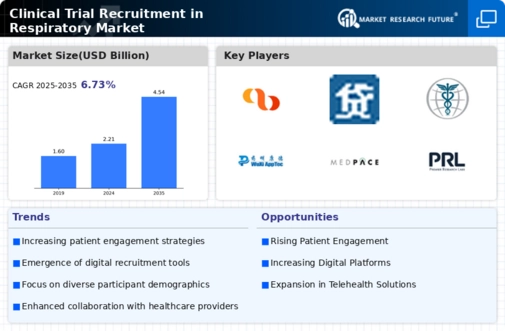
Growing Emphasis on Patient Engagement
The focus on patient engagement is reshaping the Clinical Trial Recruitment in Respiratory Market. Engaging patients throughout the trial process enhances retention rates and improves overall study outcomes. Research suggests that trials with high levels of patient engagement can achieve retention rates exceeding 80%. This shift towards a more patient-centric approach encourages sponsors to develop strategies that prioritize participant needs and preferences. By fostering open communication and providing support, researchers can create a more inviting environment for potential participants. As patient engagement becomes a cornerstone of clinical trial design, the Clinical Trial Recruitment in Respiratory Market is expected to adapt, leading to more effective recruitment and retention strategies.
Advancements in Digital Recruitment Tools
The integration of digital technologies into recruitment processes is transforming the Clinical Trial Recruitment in Respiratory Market. Tools such as social media platforms, mobile applications, and telemedicine are enhancing outreach efforts, making it easier to connect with potential participants. Data indicates that digital recruitment strategies can increase enrollment rates by up to 30%, significantly impacting the speed and efficiency of clinical trials. These advancements allow for targeted recruitment campaigns that can reach specific demographics, including those with respiratory conditions. Furthermore, the use of data analytics enables researchers to identify and engage with suitable candidates more effectively. As these technologies continue to evolve, they are expected to play a crucial role in shaping the future of clinical trial recruitment in the respiratory sector.
Increased Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, healthcare providers, and patient advocacy groups, is driving the Clinical Trial Recruitment in Respiratory Market. These partnerships facilitate knowledge sharing and resource allocation, ultimately enhancing recruitment efforts. For example, joint initiatives can lead to the development of community outreach programs that raise awareness about ongoing clinical trials. Data indicates that collaborative approaches can improve recruitment efficiency by up to 25%, as they leverage the strengths of each stakeholder. This trend towards collaboration is likely to continue, as stakeholders recognize the benefits of working together to address the challenges of recruitment in respiratory clinical trials. As a result, the Clinical Trial Recruitment in Respiratory Market may witness a more integrated and effective recruitment landscape.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies for respiratory diseases, which is influencing the Clinical Trial Recruitment in Respiratory Market. Initiatives aimed at expediting the approval process for new treatments are encouraging pharmaceutical companies to invest in clinical trials. For instance, the introduction of fast-track designations and priority review pathways has streamlined the process for promising therapies targeting respiratory conditions. This regulatory environment fosters a sense of urgency among researchers to recruit participants swiftly, as the potential for rapid market entry becomes more tangible. Consequently, the Clinical Trial Recruitment in Respiratory Market is likely to see a surge in recruitment efforts as companies strive to capitalize on these favorable conditions.
Increasing Prevalence of Respiratory Diseases
The rising incidence of respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), is a primary driver for Clinical Trial Recruitment in Respiratory Market. According to recent data, respiratory diseases affect millions worldwide, with asthma alone impacting approximately 300 million individuals. This growing patient population necessitates more clinical trials to evaluate new therapies and interventions. As the demand for innovative treatments escalates, pharmaceutical companies are increasingly focused on recruiting participants for clinical trials. The urgency to address these health challenges propels the need for effective recruitment strategies, ensuring that diverse patient populations are represented in research. Consequently, the Clinical Trial Recruitment in Respiratory Market is likely to experience heightened activity as stakeholders seek to meet the needs of this expanding demographic.