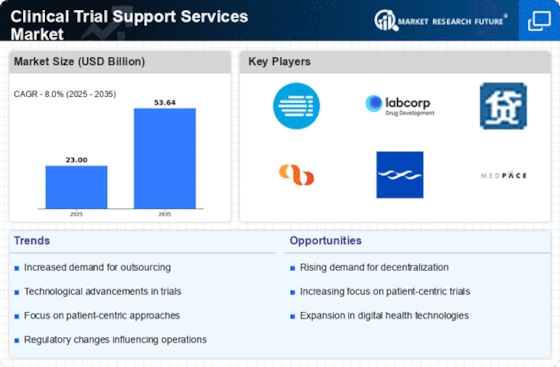
Growing Emphasis on Patient Engagement
The Clinical Trial Support Services Market is witnessing a growing emphasis on patient engagement, which is reshaping the landscape of clinical trials. Organizations are increasingly recognizing the importance of involving patients in the trial process to enhance recruitment, retention, and overall satisfaction. Strategies such as patient-centric trial designs and the use of digital platforms for communication are becoming more common. Recent surveys indicate that trials with higher levels of patient engagement tend to achieve better outcomes and faster recruitment timelines. This shift towards patient engagement not only improves the trial experience for participants but also drives the demand for specialized support services that can facilitate these initiatives, thereby enhancing the overall efficiency of clinical trials.
Rising Demand for Innovative Therapies
The Clinical Trial Support Services Market is experiencing a notable surge in demand for innovative therapies, particularly in areas such as oncology, neurology, and rare diseases. This trend is driven by the increasing prevalence of chronic diseases and the need for novel treatment options. According to recent data, the number of clinical trials initiated for new therapies has risen significantly, indicating a robust pipeline of potential treatments. As pharmaceutical companies strive to bring these innovations to market, the demand for specialized clinical trial support services, including patient recruitment and data management, is likely to grow. This shift towards innovative therapies necessitates a comprehensive approach to clinical trials, thereby enhancing the role of support services in ensuring successful outcomes.
Advancements in Data Analytics and Technology
Technological advancements, particularly in data analytics and digital health, are transforming the Clinical Trial Support Services Market. The integration of advanced data analytics tools enables more efficient trial designs, patient monitoring, and data collection processes. These innovations facilitate real-time insights, which can significantly enhance decision-making and operational efficiency in clinical trials. Furthermore, the adoption of electronic data capture (EDC) systems and mobile health applications is becoming increasingly prevalent, streamlining the management of clinical trials. As organizations leverage these technologies, the demand for clinical trial support services that specialize in data management and analytics is likely to rise, reflecting a broader trend towards data-driven decision-making in the industry.
Regulatory Changes and Compliance Requirements
The Clinical Trial Support Services Market is significantly influenced by evolving regulatory changes and compliance requirements. Regulatory bodies are continuously updating guidelines to ensure the safety and efficacy of new therapies, which impacts the design and execution of clinical trials. Organizations must navigate these complex regulations to maintain compliance, leading to an increased demand for clinical trial support services that specialize in regulatory affairs and quality assurance. Recent trends suggest that companies are investing more in compliance-related services to mitigate risks and ensure successful trial outcomes. As the regulatory landscape continues to evolve, the need for expert guidance and support in navigating these changes is likely to grow, further driving the market for clinical trial support services.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical and biotechnology sectors is a critical driver for the Clinical Trial Support Services Market. Companies are allocating substantial resources to R&D to discover and develop new drugs, which has led to an increase in the number of clinical trials. Recent statistics indicate that R&D spending has reached unprecedented levels, with many organizations prioritizing clinical trials as a key component of their growth strategies. This influx of investment not only accelerates the development of new therapies but also amplifies the demand for clinical trial support services, including regulatory compliance and trial management. As organizations seek to optimize their R&D processes, the reliance on specialized support services is expected to intensify.