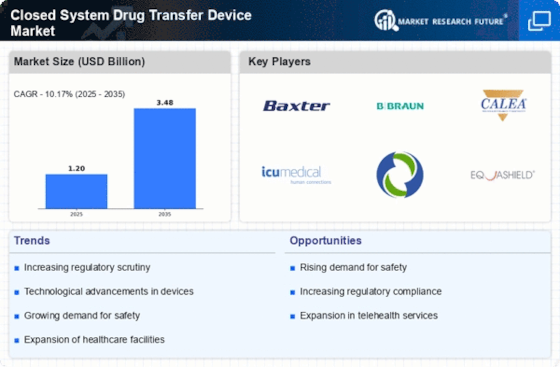
Top Industry Leaders in the Closed System Drug Transfer Device Market

 *Disclaimer: List of key companies in no particular orderLatest Closed System Drug Transfer Devices Companies Update
*Disclaimer: List of key companies in no particular orderLatest Closed System Drug Transfer Devices Companies Update
-
Oct 2023: The FDA has approved the EQUASHIELD® Syringe Unit for full-volume use, according to EQUASHIELD®, a top supplier of Closed System Transfer Devices (CSTDs) for dangerous medications. An incorporated barrier-type pressure equalization structure, a leak-prevention dry disconnection mechanism, and an enclosed closed-back syringe with a metal plunger rod are all part of the special CSTD Syringe Unit made by EQUASHIELD. These features help to reduce the risk of hazardous drug contamination by stopping vapor escape and plunger spoilage. This accomplishment marks a substantial advance for the sector, lifting the bar for security, effectiveness, and affordability. Since it has been the most widely used CSTD in the USA for five years, EQUASHIELD's extensive CSTD portfolio includes closed-back syringes and adaptors for various hazardous medication preparation and administration processes.
-
Feb 2023: The US Food & Drug Administration has granted its first product 510(k) certification to Zephyrus Innovations, a privately held medical device manufacturer and designer of safety syringes and Closed System Transfer Devices (CSTDs). This approval is for the Aeroject 3ml safety syringe. The first product in a line of safety syringes being created by Zephyrus is the Aeroject 3ml safety syringe. The need for safety syringes is rising in a market estimated to be worth $8.5 billion by 2024, with the US making up 40% of sales. Due to the potential for transferring bloodborne viruses and the growing prospect of class action lawsuits, poor safety outcomes can be both expensive and fatal. Demand for safer syringes has never been higher.
List of Closed System Drug Transfer Devices Key companies in the market
- Equashield (US)
- Simplivia Healthcare (Israel)
- Corvida Medical (US)
- Yukon Medical (US)
- CODAN Medizinische Geräte (Germany)
- VICTUS (US)
- Caragen (US)