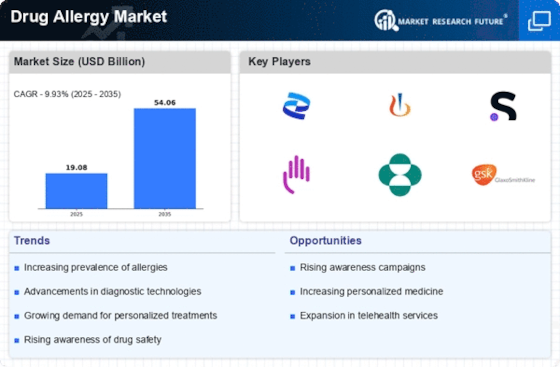
Top Industry Leaders in the Drug Allergy Market
 Latest Drug Allergy Companies Update
Latest Drug Allergy Companies Update
-
May 2023: In collaboration with Genomics England, the Medicines and Healthcare Products Regulatory Agency (MHRA) will pilot a brand-new genetic research resource called a "biobank" in order to gain a better understanding of how the genetic composition of a patient can affect the safety of their medications. In addition to the MHRA's Yellow Card reporting site, the Yellow Card biobank will house patient samples and genetic information pertaining to adverse events and suspected side effects of pharmaceuticals and medical devices. As an integral component of a comprehensive strategy towards personalized medicine, this initiative entails the utilization of the biobank's repository of genetic data by researchers to ascertain the causative factor of a medication-induced adverse effect on a particular genetic characteristic. Consequently, physicians will be empowered to tailor prescriptions to patients' genetic profiles via rapid screening tests, ensuring that individuals throughout the United Kingdom receive the safest medication possible.
-
August 2022: Glenmark Specialty S.A., a subsidiary of Glenmark Pharmaceuticals Ltd., an innovation-driven global pharmaceutical company, and Hikma Pharmaceuticals PLC, a multinational pharmaceutical company, announced in August 2022 the launch of RYALTRIS™ (olopatadine hydrochloride and mometasone furoate nasal spray) in the United States. The US Food and Drug Administration has approved RYALTRIS™ for the treatment of seasonal allergic rhinitis (SAR) symptoms in pediatric and adult patients 12 years and older. This launch furthers Hikma's goal of expanding its specialty business in the United States by capitalizing on its leadership position as one of the largest providers of nasally administered medications used to treat seasonal allergies in the United States.
List of Drug Allergy Key companies in the market
- Bayer (US)
- HAL Allergy Group (the Netherlands)
- AstraZeneca (UK)
- MAGNA Pharmaceuticals, Inc. (US)
- Circassia (US)
- Stallergenes Greer (UK)
- Johnson & Johnson Services, Inc. (US)