Rising Demand for Clinical Trials
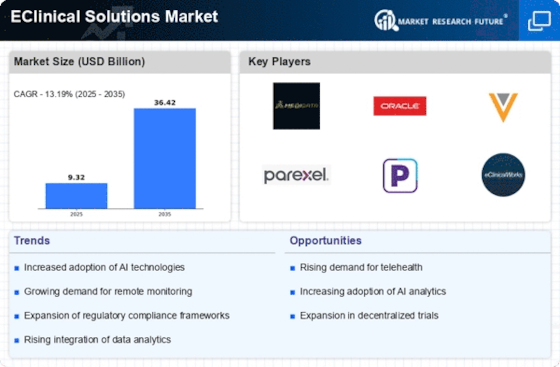
The EClinical Solutions Market is experiencing a notable increase in demand for clinical trials, driven by the need for innovative therapies and drugs. As pharmaceutical companies strive to expedite the drug development process, the reliance on eClinical solutions has surged. In 2025, the number of clinical trials initiated is projected to reach unprecedented levels, with estimates suggesting a growth rate of approximately 8% annually. This trend indicates a robust market for eClinical solutions, as organizations seek to streamline trial management, enhance data collection, and improve patient engagement. The integration of advanced technologies within the EClinical Solutions Market is likely to facilitate more efficient trial designs and execution, ultimately leading to faster time-to-market for new treatments.
Increased Focus on Patient Engagement
The EClinical Solutions Market is witnessing a paradigm shift towards enhanced patient engagement in clinical trials. Organizations are recognizing the importance of involving patients in the research process to improve retention rates and data quality. In 2025, it is projected that patient-centric approaches will account for a significant portion of trial designs, with an estimated 40% of trials incorporating digital tools for patient engagement. This trend is likely to drive the demand for eClinical solutions that facilitate communication, education, and feedback from participants. By leveraging technology to enhance patient experiences, the EClinical Solutions Market may see improved outcomes and more efficient trial processes.
Regulatory Support for Digital Solutions
Regulatory bodies are increasingly supporting the adoption of digital solutions within the EClinical Solutions Market. Recent initiatives aimed at modernizing regulatory frameworks have paved the way for the integration of electronic systems in clinical trials. In 2025, it is expected that more than 50% of regulatory submissions will utilize electronic formats, reflecting a shift towards digitalization. This regulatory support not only streamlines the approval process but also encourages organizations to invest in eClinical solutions that comply with evolving standards. As the regulatory landscape continues to adapt, the EClinical Solutions Market is likely to benefit from increased confidence in digital methodologies, fostering innovation and efficiency.
Growing Need for Real-Time Data Analytics
The demand for real-time data analytics is becoming increasingly critical within the EClinical Solutions Market. As clinical trials become more complex, the ability to analyze data in real-time is essential for making informed decisions and ensuring trial integrity. In 2025, it is projected that the market for real-time analytics solutions will grow by approximately 15%, driven by the need for timely insights into trial performance and patient safety. Organizations are recognizing that traditional data analysis methods may not suffice in the fast-paced clinical environment. Consequently, the EClinical Solutions Market is likely to see a surge in the adoption of advanced analytics tools that provide actionable insights, thereby enhancing trial efficiency and outcomes.
Technological Advancements in Data Management
Technological advancements are significantly shaping the EClinical Solutions Market, particularly in data management. The emergence of cloud-based platforms and advanced analytics tools has revolutionized how clinical data is collected, stored, and analyzed. In 2025, it is anticipated that over 60% of clinical data will be managed through cloud solutions, enhancing accessibility and collaboration among stakeholders. These innovations not only improve data integrity but also enable real-time monitoring of clinical trials, which is crucial for timely decision-making. As organizations increasingly adopt these technologies, the EClinical Solutions Market is likely to witness a surge in demand for integrated data management solutions that can support complex trial designs and regulatory requirements.
















Leave a Comment