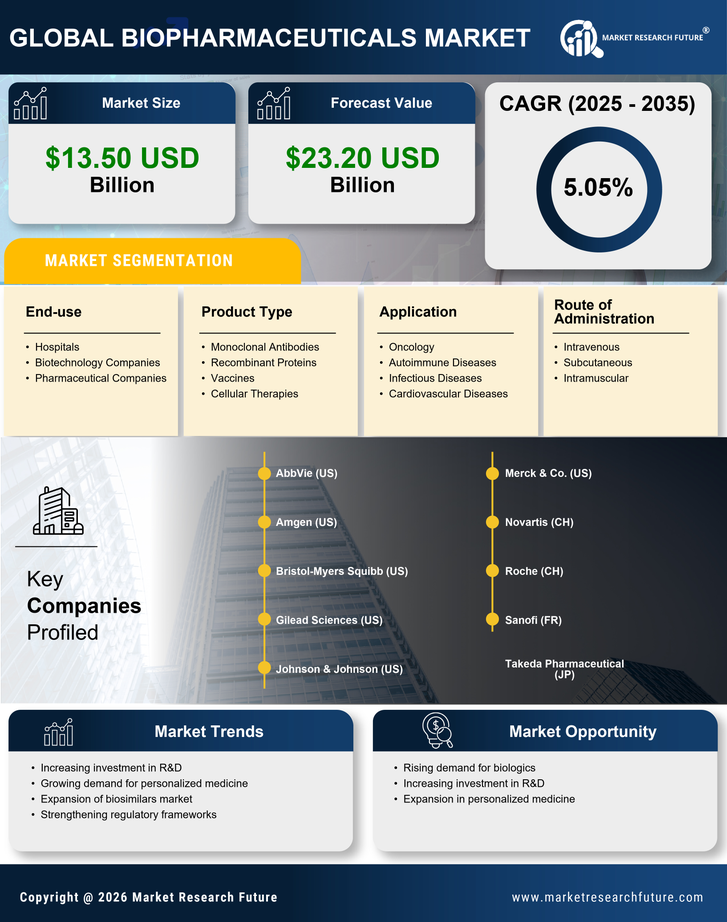
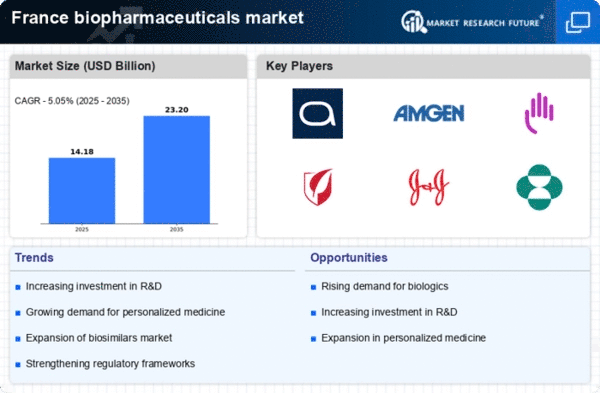
Advancements in Biotechnology
Technological advancements in biotechnology are significantly influencing the biopharmaceuticals market in France. Innovations such as CRISPR gene editing and monoclonal antibody development are paving the way for new therapeutic options. The French biotechnology sector is expected to grow at a CAGR of 10% over the next five years, driven by increased collaboration between research institutions and biopharmaceutical companies. These advancements not only enhance the efficacy of treatments but also reduce production costs, making therapies more accessible. As a result, the biopharmaceuticals market is likely to see a surge in novel products that leverage these cutting-edge technologies.
Rising Healthcare Expenditure
Healthcare expenditure in France is on the rise, which is positively impacting the biopharmaceuticals market. In 2025, total healthcare spending is projected to reach €300 billion, with a significant portion allocated to pharmaceuticals. This increase in funding allows for greater access to innovative biopharmaceutical products, as well as improved patient care. The French government is also focusing on enhancing healthcare infrastructure, which further supports the growth of the biopharmaceuticals market. As more resources are directed towards healthcare, the demand for advanced therapies is likely to escalate, driving market expansion.
Growing Demand for Targeted Therapies
The biopharmaceuticals market in France is experiencing a notable increase in demand for targeted therapies. This trend is driven by the rising prevalence of chronic diseases and the need for more effective treatment options. In 2025, the market for targeted therapies is projected to reach approximately €5 billion, reflecting a growth rate of around 8% annually. Patients and healthcare providers are increasingly favoring therapies that offer personalized treatment plans, which are often more effective and have fewer side effects. This shift towards precision medicine is reshaping the landscape of the biopharmaceuticals market, as companies invest in developing innovative solutions tailored to individual patient needs.
Collaboration Between Public and Private Sectors
Collaboration between public and private sectors is emerging as a vital driver for the biopharmaceuticals market in France. Partnerships between government agencies, research institutions, and biopharmaceutical companies are fostering innovation and accelerating the development of new therapies. In 2025, it is expected that such collaborations will lead to the introduction of at least 20 new biopharmaceutical products. These alliances not only enhance research capabilities but also facilitate the sharing of resources and expertise, ultimately benefiting the biopharmaceuticals market. This collaborative approach is likely to yield significant advancements in treatment options and improve patient outcomes.
Regulatory Support for Biopharmaceutical Innovations
The regulatory environment in France is becoming increasingly supportive of biopharmaceutical innovations, which is a crucial driver for the market. The French government has implemented policies aimed at expediting the approval process for new drugs, particularly those that address unmet medical needs. In 2025, it is anticipated that the number of new biopharmaceutical products approved will increase by 15%, fostering a more dynamic market landscape. This regulatory support not only encourages investment in research and development but also enhances the competitiveness of the biopharmaceuticals market on an international scale.