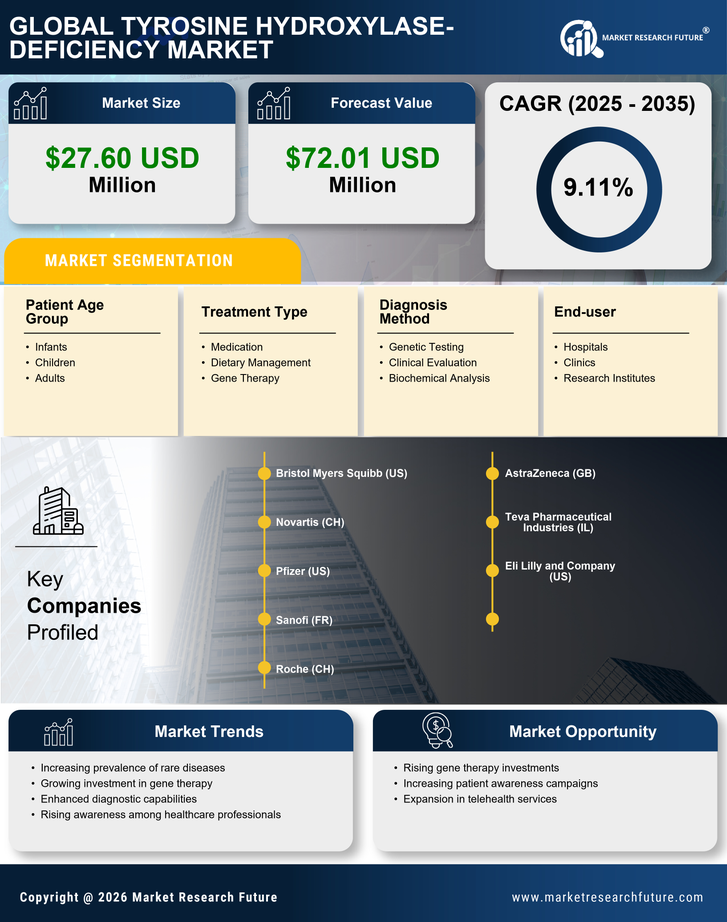
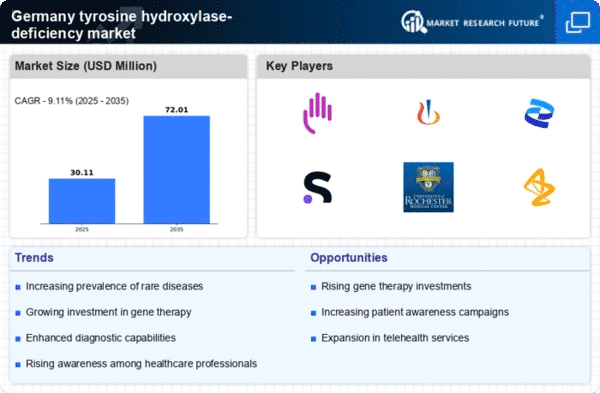
Advancements in Genetic Testing
Technological advancements in genetic testing are significantly impacting the tyrosine hydroxylase-deficiency market. In Germany, the availability of next-generation sequencing (NGS) has revolutionized the diagnosis of rare genetic disorders, allowing for earlier and more accurate identification of tyrosine hydroxylase deficiency. This has led to an increase in diagnosed cases, which in turn drives demand for targeted therapies and management strategies. The market is expected to grow as healthcare providers increasingly adopt these advanced testing methods, facilitating timely interventions. Moreover, the integration of genetic counseling services alongside testing is likely to enhance patient outcomes, further propelling the growth of the tyrosine hydroxylase-deficiency market.
Growing Patient Advocacy Groups
The emergence of patient advocacy groups in Germany is playing a pivotal role in shaping the tyrosine hydroxylase-deficiency market. These organizations are instrumental in raising awareness about the condition, providing support to affected families, and lobbying for better healthcare policies. Their efforts have led to increased visibility of tyrosine hydroxylase deficiency, which may encourage more individuals to seek diagnosis and treatment. Furthermore, advocacy groups often collaborate with researchers and pharmaceutical companies, facilitating clinical trials and the development of new therapies. This collaborative environment is likely to foster innovation within the tyrosine hydroxylase-deficiency market, ultimately benefiting patients and healthcare providers alike.
Regulatory Framework Enhancements
The regulatory landscape in Germany is evolving to better support the development of treatments for rare diseases, including tyrosine hydroxylase deficiency. Recent enhancements in regulatory frameworks aim to streamline the approval process for orphan drugs, which are essential for addressing the needs of patients with rare conditions. These changes may reduce the time and cost associated with bringing new therapies to market, thereby encouraging pharmaceutical companies to invest in the development of treatments for tyrosine hydroxylase deficiency. As a result, the tyrosine hydroxylase-deficiency market is likely to experience growth, with more innovative therapies becoming available to patients in the near future.
Rising Incidence of Neurological Disorders
The increasing prevalence of neurological disorders in Germany is a crucial driver for the tyrosine hydroxylase-deficiency market. Recent studies indicate that the incidence of rare genetic disorders, including tyrosine hydroxylase deficiency, is on the rise, with estimates suggesting that approximately 1 in 100,000 live births may be affected. This growing patient population necessitates the development of specialized treatments and therapies, thereby expanding the market. Furthermore, as awareness of these conditions increases among healthcare professionals and the public, the demand for diagnostic and therapeutic solutions is likely to grow. The tyrosine hydroxylase-deficiency market is thus positioned to benefit from this trend, as healthcare systems adapt to meet the needs of an expanding patient demographic.
Increased Investment in Rare Disease Research
Investment in research and development for rare diseases is a significant driver for the tyrosine hydroxylase-deficiency market. In Germany, both public and private sectors are allocating substantial funds towards understanding and treating rare genetic disorders. Reports indicate that funding for rare disease research has increased by over 30% in recent years, reflecting a growing recognition of the need for effective therapies. This influx of capital is likely to accelerate the development of innovative treatments for tyrosine hydroxylase deficiency, enhancing the market landscape. As more pharmaceutical companies enter this space, competition may lead to improved treatment options and potentially lower costs for patients.