Advancements in Genetic Testing
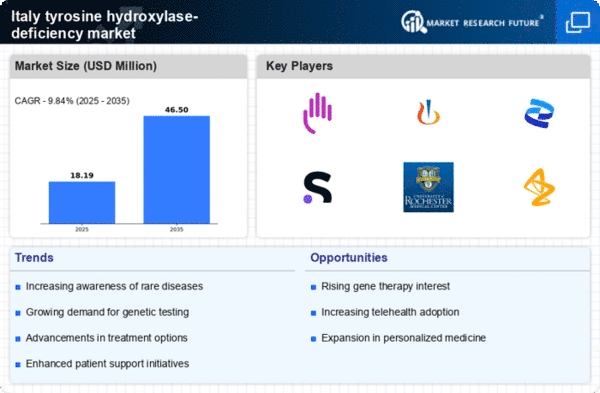
Technological advancements in genetic testing are transforming the landscape of the tyrosine hydroxylase-deficiency market in Italy. The introduction of next-generation sequencing (NGS) has significantly improved the accuracy and speed of diagnosing genetic disorders, including tyrosine hydroxylase deficiency. As a result, healthcare providers are increasingly able to identify affected individuals earlier, which is crucial for timely intervention. The market for genetic testing in Italy is projected to grow at a CAGR of around 10% over the next five years, driven by the rising demand for precision medicine. This growth is likely to enhance the overall understanding of tyrosine hydroxylase deficiency, leading to more targeted therapies and better management strategies. Consequently, the advancements in genetic testing are expected to play a pivotal role in shaping the future of the tyrosine hydroxylase-deficiency market.
Regulatory Framework Enhancements
The regulatory landscape in Italy is evolving to better accommodate the needs of the tyrosine hydroxylase-deficiency market. Recent reforms aimed at expediting the approval process for orphan drugs are likely to facilitate faster access to innovative therapies for patients. The Italian Medicines Agency (AIFA) has implemented measures to streamline the evaluation of treatments for rare diseases, which could significantly reduce the time from development to market. This regulatory support is crucial, as it encourages pharmaceutical companies to invest in research and development for tyrosine hydroxylase deficiency. As a result, the market may witness an influx of new therapies, improving treatment options for patients. Furthermore, these regulatory enhancements may foster a more competitive environment, ultimately benefiting patients through increased access to effective treatments.
Rising Incidence of Neurological Disorders
The increasing prevalence of neurological disorders in Italy is a significant driver for the tyrosine hydroxylase-deficiency market. Recent studies indicate that the incidence of rare genetic disorders, including tyrosine hydroxylase deficiency, is on the rise, with estimates suggesting that approximately 1 in 100,000 live births may be affected. This growing patient population necessitates enhanced diagnostic and therapeutic options, thereby stimulating market growth. Furthermore, as awareness of these conditions expands among healthcare professionals and the public, the demand for specialized treatments is likely to increase. The Italian healthcare system is adapting to these needs, potentially leading to more funding and resources allocated to research and development in the tyrosine hydroxylase-deficiency market. This trend may result in a more robust pipeline of therapies and improved patient outcomes in the coming years.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups in Italy is significantly influencing the tyrosine hydroxylase-deficiency market. These organizations play a crucial role in raising awareness about the condition, providing resources for affected families, and advocating for better healthcare policies. As these groups gain traction, they are likely to enhance the visibility of tyrosine hydroxylase deficiency, leading to increased funding for research and improved access to treatments. In recent years, the number of active support groups has increased by approximately 30%, indicating a growing community of advocates. This trend may encourage pharmaceutical companies to invest in the development of new therapies, as the demand for effective treatments becomes more pronounced. Consequently, the presence of these advocacy groups is expected to have a lasting impact on the tyrosine hydroxylase-deficiency market.
Increased Investment in Rare Disease Research
The tyrosine hydroxylase-deficiency market is experiencing a surge in investment focused on rare disease research in Italy. Government initiatives and private sector funding are increasingly directed towards understanding and treating rare genetic disorders. In 2025, it is estimated that funding for rare disease research in Italy could reach €500 million, reflecting a growing commitment to addressing these conditions. This influx of capital is likely to facilitate the development of innovative therapies and improve access to existing treatments. Additionally, collaborations between academic institutions and pharmaceutical companies are becoming more common, fostering an environment conducive to breakthroughs in the tyrosine hydroxylase-deficiency market. As research progresses, it is anticipated that new treatment options will emerge, further driving market growth and enhancing patient care.