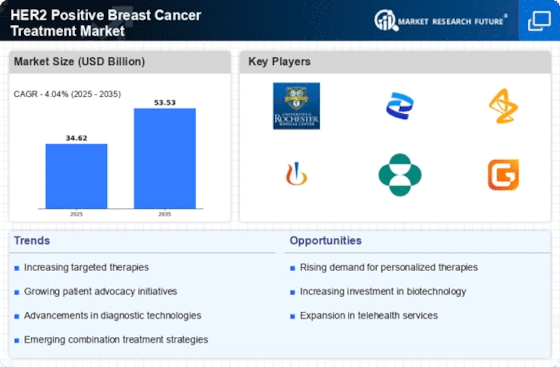
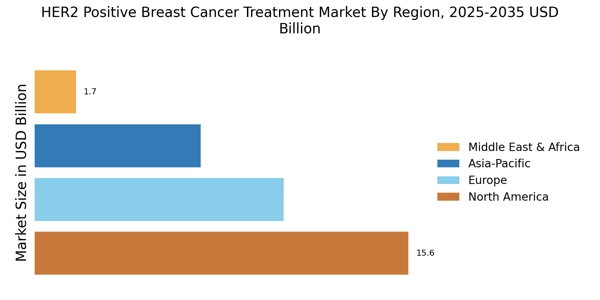
Advancements in Targeted Therapies
Advancements in targeted therapies represent a significant driver for the HER2 Positive Breast Cancer Treatment Market. The introduction of monoclonal antibodies and tyrosine kinase inhibitors has revolutionized treatment protocols, offering improved outcomes for patients. For instance, drugs such as trastuzumab and pertuzumab have demonstrated substantial efficacy in clinical trials, leading to their widespread adoption. The market for HER2 targeted therapies is projected to reach several billion dollars by 2026, reflecting the growing reliance on precision medicine. These advancements not only enhance survival rates but also reduce the side effects associated with traditional chemotherapy, making targeted therapies a preferred choice among oncologists and patients alike.
Increased Investment in Cancer Research
Increased investment in cancer research is a crucial driver for the HER2 Positive Breast Cancer Treatment Market. Governments and private organizations are allocating significant funds to explore novel treatment modalities and improve existing therapies. This influx of capital is fostering innovation in drug development, particularly for HER2 positive breast cancer. For example, funding initiatives have led to the exploration of combination therapies that enhance the efficacy of existing treatments. As research progresses, new therapeutic options are likely to emerge, further stimulating market growth. The commitment to advancing cancer research underscores the importance of addressing the needs of HER2 positive patients, thereby reinforcing the market's potential.
Growing Awareness and Education Initiatives
Growing awareness and education initiatives regarding HER2 positive breast cancer are driving the HER2 Positive Breast Cancer Treatment Market. Campaigns aimed at educating both healthcare professionals and the general public about the disease are crucial in promoting early detection and treatment. Increased knowledge about HER2 positivity and its implications encourages patients to seek timely medical advice and appropriate therapies. As awareness rises, the demand for specialized treatments is likely to increase, leading to a more robust market. Additionally, educational programs that highlight the importance of genetic testing and personalized medicine are expected to further enhance patient engagement and treatment adherence.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a vital driver for the HER2 Positive Breast Cancer Treatment Market. Regulatory agencies are increasingly expediting the approval processes for new drugs, particularly those targeting HER2 positive breast cancer. This trend is evident in the accelerated approval pathways established for breakthrough therapies, which aim to bring effective treatments to market more swiftly. As a result, pharmaceutical companies are more inclined to invest in research and development for HER2 targeted therapies, knowing that regulatory hurdles may be minimized. This supportive environment not only fosters innovation but also enhances patient access to cutting-edge treatments, thereby propelling market growth.
Rising Incidence of HER2 Positive Breast Cancer
The increasing incidence of HER2 positive breast cancer is a primary driver for the HER2 Positive Breast Cancer Treatment Market. Recent statistics indicate that approximately 20 to 25% of breast cancer cases are HER2 positive, which translates to a substantial patient population requiring targeted therapies. This rising prevalence necessitates the development and availability of effective treatment options, thereby propelling market growth. Furthermore, as awareness regarding breast cancer screening and genetic testing improves, more cases are being diagnosed at earlier stages. This trend is likely to enhance the demand for innovative therapies tailored specifically for HER2 positive patients, thereby expanding the market landscape.