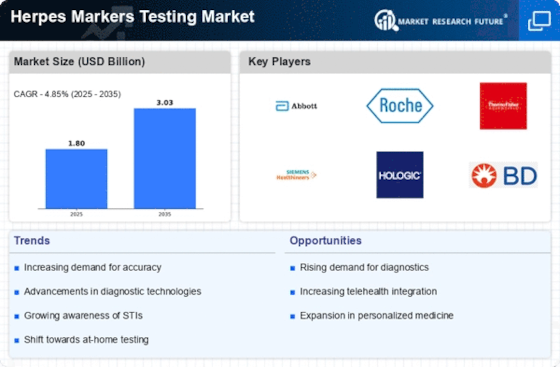
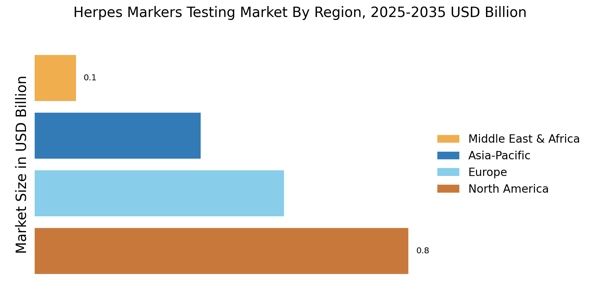
Regulatory Support and Guidelines
Regulatory support and the establishment of guidelines for herpes testing are significant drivers for the Herpes Markers Testing Market. Health authorities are increasingly recognizing the importance of standardized testing protocols to ensure accurate diagnosis and treatment of herpes infections. This regulatory framework not only enhances the credibility of testing methods but also encourages healthcare providers to adopt recommended practices. As guidelines evolve to reflect the latest scientific evidence, the market is likely to see an increase in the adoption of herpes markers testing. Additionally, regulatory bodies are promoting awareness campaigns that emphasize the importance of testing, further driving demand. This supportive environment is expected to foster growth within the Herpes Markers Testing Market, as stakeholders align with established standards to improve patient care.
Growing Focus on Preventive Healthcare
The increasing emphasis on preventive healthcare is a notable driver for the Herpes Markers Testing Market. As healthcare systems worldwide shift towards preventive measures, the importance of early detection and management of sexually transmitted infections (STIs) becomes paramount. This focus encourages individuals to undergo regular testing for herpes markers, thereby facilitating timely intervention and reducing transmission rates. Public health campaigns aimed at educating populations about the risks associated with herpes infections further support this trend. Additionally, healthcare providers are advocating for routine screenings, particularly among high-risk groups, which is likely to bolster the demand for herpes markers testing. This proactive approach to health management is expected to significantly contribute to the growth of the Herpes Markers Testing Market.
Rising Prevalence of Herpes Infections
The increasing prevalence of herpes simplex virus (HSV) infections is a primary driver for the Herpes Markers Testing Market. Recent estimates suggest that a significant portion of the population is affected by HSV-1 and HSV-2, with millions of new cases reported annually. This rising incidence necessitates effective testing solutions to facilitate early diagnosis and management of the condition. As awareness of the health implications associated with herpes infections grows, individuals are more likely to seek testing services. Consequently, the demand for herpes markers testing is expected to rise, propelling market growth. Furthermore, healthcare providers are increasingly recommending routine testing, particularly for at-risk populations, which further underscores the importance of the Herpes Markers Testing Market in addressing public health needs.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic testing are significantly influencing the Herpes Markers Testing Market. The development of more sensitive and specific testing methods, such as polymerase chain reaction (PCR) and serological assays, has enhanced the accuracy of herpes diagnosis. These advancements not only improve patient outcomes but also increase the efficiency of testing processes. As a result, healthcare providers are more inclined to adopt these advanced testing solutions, leading to a surge in demand within the market. Moreover, the integration of point-of-care testing devices allows for rapid results, which is particularly appealing in urgent care settings. This trend indicates a shift towards more accessible and efficient testing options, thereby driving the growth of the Herpes Markers Testing Market.
Increased Investment in Healthcare Infrastructure
The surge in investment in healthcare infrastructure is positively impacting the Herpes Markers Testing Market. Governments and private entities are allocating substantial resources to enhance laboratory capabilities and expand testing facilities. This investment is crucial for improving access to herpes testing services, particularly in underserved regions. Enhanced infrastructure not only facilitates the availability of advanced testing technologies but also ensures that healthcare providers can meet the growing demand for herpes markers testing. Furthermore, the establishment of specialized clinics and testing centers dedicated to STIs is likely to increase awareness and encourage individuals to seek testing. As a result, the Herpes Markers Testing Market stands to benefit from these infrastructural developments, which are essential for addressing the rising burden of herpes infections.