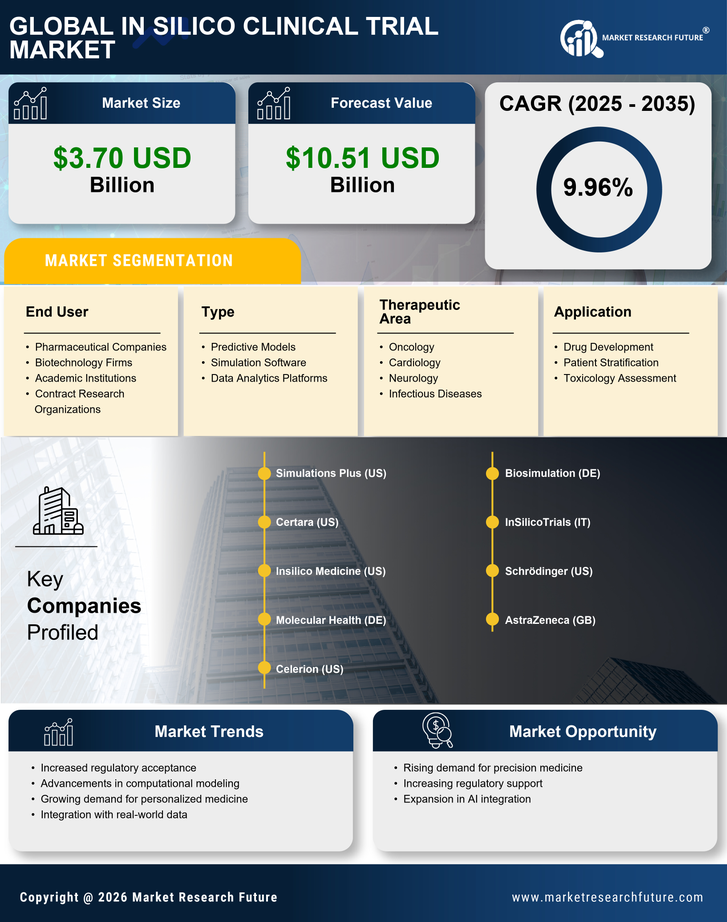
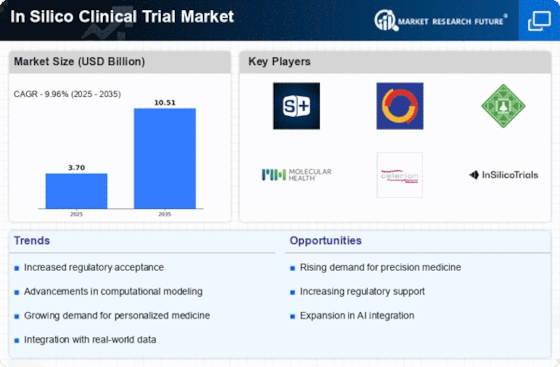
Regulatory Support and Frameworks
The In Silico Clinical Trial Market is witnessing a growing trend of regulatory support and the establishment of frameworks that facilitate the acceptance of in silico methodologies. Regulatory bodies are increasingly recognizing the potential of computational models to complement traditional clinical trials. For instance, the FDA has issued guidance on the use of modeling and simulation in drug development, which encourages the adoption of in silico trials. This regulatory endorsement is crucial, as it provides a clear pathway for companies to validate their in silico approaches. As more regulatory frameworks are developed, the confidence in silico trials is likely to increase, further propelling the growth of the In Silico Clinical Trial Market.
Cost Efficiency in Drug Development
The In Silico Clinical Trial Market is experiencing a notable shift towards cost efficiency in drug development processes. Traditional clinical trials are often prohibitively expensive, with estimates suggesting that the average cost of bringing a new drug to market can exceed 2.6 billion USD. In contrast, in silico trials utilize computational models to simulate clinical scenarios, potentially reducing costs by up to 30%. This financial incentive is driving pharmaceutical companies to adopt in silico methodologies, as they seek to streamline their research and development efforts while maintaining compliance with regulatory standards. The ability to conduct virtual trials not only minimizes resource expenditure but also accelerates the timeline for drug approval, making it an attractive option for stakeholders in the In Silico Clinical Trial Market.
Enhanced Data Analytics Capabilities
The In Silico Clinical Trial Market is increasingly benefiting from enhanced data analytics capabilities. With the advent of advanced computational techniques and machine learning algorithms, researchers can analyze vast datasets more effectively than ever before. This capability allows for the identification of patient populations that are most likely to respond to specific treatments, thereby optimizing trial designs. Reports indicate that the integration of sophisticated analytics can improve the predictive accuracy of trial outcomes by as much as 40%. As a result, pharmaceutical companies are more inclined to invest in silico trials, as they can leverage these insights to make informed decisions, reduce trial failures, and ultimately bring safer and more effective drugs to market.
Accelerated Drug Development Timelines
The In Silico Clinical Trial Market is characterized by accelerated drug development timelines, a critical factor in the competitive pharmaceutical landscape. Traditional clinical trials can take several years to complete, often delaying the introduction of new therapies to the market. In contrast, in silico trials can significantly shorten these timelines by allowing for rapid simulations and iterative testing of hypotheses. Current estimates suggest that in silico methodologies can reduce the time to market by up to 50%. This acceleration is particularly beneficial in therapeutic areas where time is of the essence, such as infectious diseases and chronic conditions. As companies strive to meet market demands swiftly, the ability to conduct faster trials through in silico methods is becoming increasingly attractive, thereby driving growth in the In Silico Clinical Trial Market.
Rising Demand for Personalized Medicine
The In Silico Clinical Trial Market is significantly influenced by the rising demand for personalized medicine. As healthcare shifts towards more individualized treatment approaches, the need for tailored clinical trials becomes paramount. In silico trials offer the flexibility to model various patient responses based on genetic, environmental, and lifestyle factors. This adaptability is particularly appealing in oncology and rare diseases, where patient heterogeneity can complicate traditional trial designs. The market for personalized medicine is projected to reach 2.4 trillion USD by 2025, indicating a robust opportunity for in silico methodologies to play a pivotal role in developing targeted therapies. Consequently, the integration of personalized medicine principles into in silico trials is likely to enhance their relevance and application within the In Silico Clinical Trial Market.