Advancements in Biotechnology
Biotechnology innovations are significantly influencing the multiplex assays market in Japan. The development of novel reagents, improved detection technologies, and enhanced data analysis software are facilitating the creation of more sophisticated multiplex assays. These advancements allow for higher sensitivity and specificity in detecting various diseases, which is crucial for accurate diagnostics. Furthermore, the integration of artificial intelligence and machine learning in assay development is streamlining processes and improving outcomes. As a result, the multiplex assays market is likely to expand, with an anticipated market value of $600 million by 2026, driven by these technological enhancements.
Enhanced Regulatory Framework
The regulatory framework surrounding diagnostic tools in Japan is evolving, which is likely to benefit the multiplex assays market. The Japanese government is implementing streamlined approval processes for innovative diagnostic technologies, thereby encouraging the development and commercialization of multiplex assays. This supportive regulatory environment is expected to foster innovation and increase market entry for new products. As a result, the multiplex assays market may experience accelerated growth, with an estimated market size of $650 million by 2027. The enhanced regulatory framework is crucial for ensuring that high-quality multiplex assays are available to healthcare providers and patients.
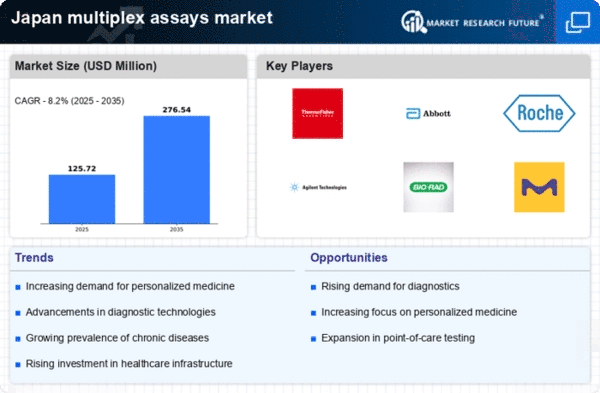
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in Japan is a pivotal driver for the multiplex assays market. As the population ages, conditions such as diabetes, cardiovascular diseases, and cancer are becoming more prevalent. This trend necessitates advanced diagnostic tools that can provide comprehensive insights into multiple biomarkers simultaneously. The multiplex assays market is expected to benefit from this demand, as these assays enable healthcare providers to conduct efficient screenings and monitor disease progression. In 2025, the market is projected to reach approximately $500 million, reflecting a compound annual growth rate (CAGR) of around 10%. This growth underscores the critical role that multiplex assays play in managing chronic diseases effectively.
Rising Awareness of Preventive Healthcare
There is a growing awareness of preventive healthcare among the Japanese population, which is positively impacting the multiplex assays market. As individuals become more health-conscious, there is an increasing demand for early detection and preventive measures for various diseases. Multiplex assays offer a practical solution by enabling simultaneous testing for multiple conditions, thus facilitating timely interventions. This trend is expected to drive market growth, with projections indicating that the multiplex assays market could reach $550 million by 2026. The emphasis on preventive healthcare aligns with national health policies aimed at reducing the burden of diseases.
Growing Investment in Research and Development
Investment in research and development (R&D) within Japan's healthcare sector is a significant driver for the multiplex assays market. The government and private entities are increasingly allocating funds to develop innovative diagnostic solutions. This focus on R&D is fostering collaborations between academic institutions and industry players, leading to the creation of cutting-edge multiplex assays. In 2025, R&D spending in the healthcare sector is expected to exceed $10 billion, with a substantial portion directed towards diagnostic technologies. This investment is likely to enhance the capabilities of multiplex assays, making them more accessible and effective in clinical settings.