Advancements in Biotechnology
Technological innovations in biotechnology are significantly influencing the multiplex assays market. The development of novel assay platforms and reagents enhances the sensitivity and specificity of tests, making them more reliable for clinical applications. In South Korea, research institutions and biotech companies are investing heavily in R&D to create cutting-edge multiplex assays. This investment is likely to lead to the introduction of new products that cater to diverse diagnostic needs. The market is expected to expand as these advancements facilitate the integration of multiplex assays into routine diagnostics, potentially increasing their adoption rate by 15% over the next few years. The continuous evolution of biotechnology thus appears to be a driving force behind the growth of the multiplex assays market.
Supportive Government Policies
Government policies in South Korea are increasingly supportive of the multiplex assays market. Initiatives aimed at enhancing healthcare infrastructure and promoting innovative diagnostic technologies are being implemented. The government is likely to provide funding and incentives for research and development in the field of diagnostics, which could lead to the emergence of new multiplex assay technologies. Furthermore, regulatory frameworks are being streamlined to facilitate the approval process for new diagnostic tests. This supportive environment is expected to foster growth in the multiplex assays market, with projections indicating a potential market size increase to $400 million by 2028. Such policies are crucial for encouraging innovation and ensuring that advanced diagnostic tools are accessible to healthcare providers.
Rising Demand for Point-of-Care Testing
The demand for point-of-care testing (POCT) is on the rise in South Korea, significantly impacting the multiplex assays market. POCT allows for rapid diagnosis and immediate clinical decision-making, which is particularly beneficial in emergency and outpatient settings. Multiplex assays that can be performed at the point of care are increasingly sought after, as they provide timely results without the need for extensive laboratory infrastructure. This trend is likely to drive market growth, with estimates suggesting that the POCT segment of the multiplex assays market could grow by 25% over the next five years. The convenience and efficiency of point-of-care testing thus represent a compelling driver for the multiplex assays market.
Growing Focus on Early Disease Detection
The emphasis on early disease detection in South Korea is propelling the multiplex assays market forward. Healthcare professionals increasingly recognize that early diagnosis can lead to better patient outcomes and lower healthcare costs. Multiplex assays, which allow for the simultaneous detection of multiple pathogens or biomarkers, are particularly valuable in this context. The market is likely to benefit from initiatives aimed at promoting preventive healthcare measures. As awareness of the importance of early detection grows, the demand for multiplex assays is expected to rise, potentially increasing market revenues by 20% by 2027. This focus on proactive healthcare strategies is thus a significant driver for the multiplex assays market.
Increasing Prevalence of Chronic Diseases
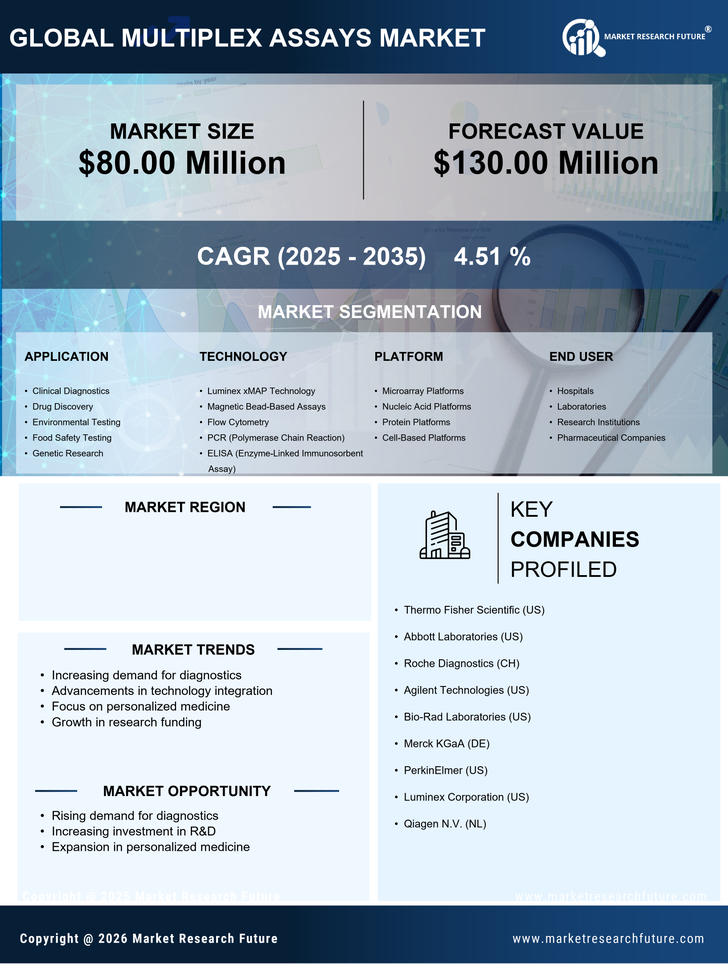
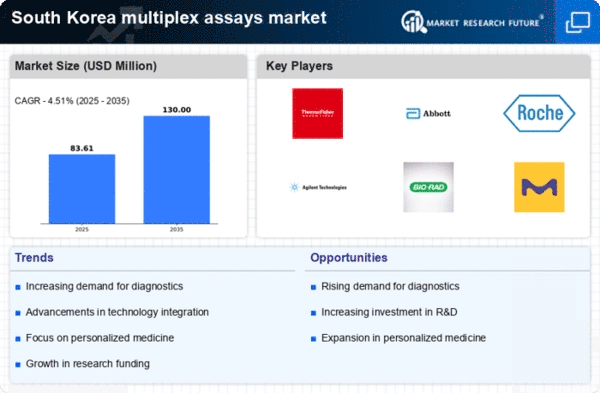
The rising incidence of chronic diseases in South Korea is a pivotal driver for the multiplex assays market. As the population ages, conditions such as diabetes, cardiovascular diseases, and cancer are becoming more prevalent. This trend necessitates advanced diagnostic tools that can provide rapid and accurate results. Multiplex assays, capable of detecting multiple biomarkers simultaneously, are increasingly favored in clinical settings. The market is projected to grow as healthcare providers seek efficient solutions to manage these diseases. In 2025, the market for multiplex assays is expected to reach approximately $300 million, reflecting a compound annual growth rate (CAGR) of around 10%. This growth underscores the critical role of multiplex assays in addressing the healthcare challenges posed by chronic diseases.