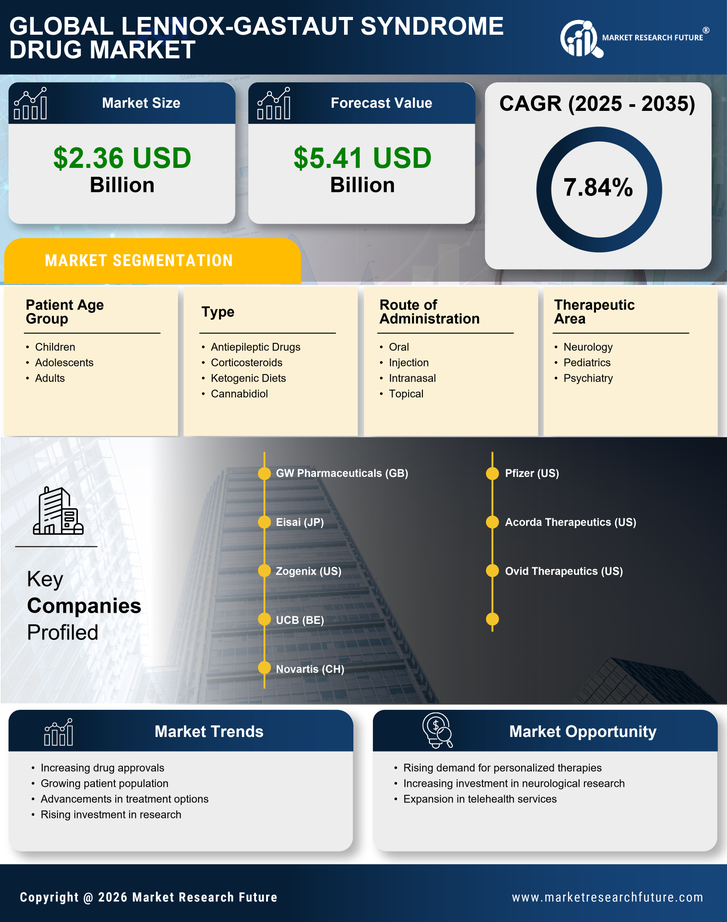
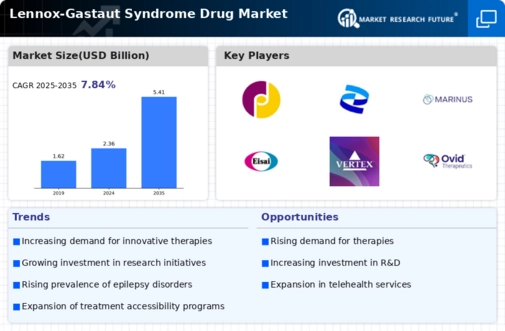
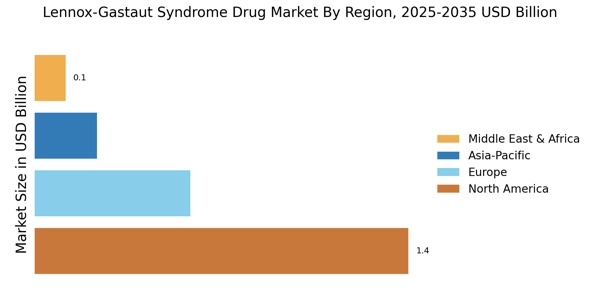
Regulatory Incentives for Drug Approval
Regulatory bodies are providing incentives to encourage the development of treatments for Lennox-Gastaut Syndrome, which is positively impacting the Lennox-Gastaut Syndrome Drug Market. Programs such as orphan drug designation and fast track approval are designed to expedite the review process for medications targeting rare diseases. These initiatives not only reduce the time required for drug approval but also offer financial benefits to developers, making it more attractive to invest in LGS therapies. As a result, the regulatory landscape is becoming increasingly favorable for companies operating within the Lennox-Gastaut Syndrome Drug Market, potentially leading to a more robust pipeline of innovative treatments.
Growing Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, academic institutions, and patient advocacy groups, is emerging as a key driver in the Lennox-Gastaut Syndrome Drug Market. These partnerships are fostering knowledge sharing and resource pooling, which can accelerate the development of new therapies. Joint initiatives often lead to more comprehensive research efforts, addressing the multifaceted nature of LGS. Additionally, collaborations can enhance patient recruitment for clinical trials, thereby expediting the drug development process. As the Lennox-Gastaut Syndrome Drug Market continues to evolve, these collaborative efforts are likely to play a crucial role in bringing innovative treatments to market more efficiently.
Rising Prevalence of Lennox-Gastaut Syndrome
The increasing incidence of Lennox-Gastaut Syndrome (LGS) is a notable driver for the Lennox-Gastaut Syndrome Drug Market. Recent estimates suggest that LGS affects approximately 1 in 50,000 children, leading to a growing demand for effective treatment options. As awareness of this condition rises among healthcare professionals and families, the need for specialized medications becomes more pronounced. This trend is likely to stimulate research and development efforts, resulting in a wider array of therapeutic options. Furthermore, the rising prevalence may encourage pharmaceutical companies to invest in the Lennox-Gastaut Syndrome Drug Market, potentially leading to innovative solutions that address the unique challenges posed by this complex epilepsy syndrome.
Advancements in Drug Development Technologies
Technological advancements in drug development are significantly influencing the Lennox-Gastaut Syndrome Drug Market. Innovations such as high-throughput screening and personalized medicine are enabling researchers to identify and develop new therapeutic agents more efficiently. These technologies facilitate the discovery of drugs that target specific pathways involved in LGS, potentially improving treatment outcomes. Moreover, the integration of artificial intelligence in drug discovery processes is streamlining the identification of promising candidates, thereby accelerating the time to market. As these advancements continue to evolve, they are likely to enhance the competitiveness of the Lennox-Gastaut Syndrome Drug Market, attracting further investment and fostering collaboration among stakeholders.
Increased Investment in Rare Disease Research
The growing focus on rare diseases, including Lennox-Gastaut Syndrome, is driving investment in the Lennox-Gastaut Syndrome Drug Market. Pharmaceutical companies and research institutions are increasingly recognizing the unmet medical needs associated with LGS, leading to a surge in funding for research initiatives. In recent years, the market has seen a notable increase in venture capital investments aimed at developing novel therapies for rare conditions. This influx of capital is expected to facilitate the development of innovative drugs, thereby expanding the treatment landscape for LGS. As more stakeholders enter the Lennox-Gastaut Syndrome Drug Market, competition may intensify, ultimately benefiting patients through improved access to effective therapies.