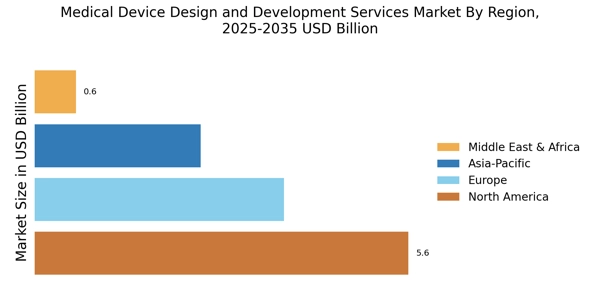
By region, the study provides the market insights into North America, Europe, Asia-Pacific and Rest of the World. North America has captured the largest market share in the Medical Device Design and Development Services Market due to several factors. The region boasts a robust healthcare infrastructure, a large base of medical device companies, and significant investments in research and development. Additionally, favorable government policies, advanced technological capabilities, and a supportive regulatory environment contribute to North America's dominance in the market.
Furthermore, the presence of key players and academic institutions specializing in medical device innovation further strengthens North America's position as a leader in medical device design and development services.
Further, the major countries studied in the market report are the US, Canada, Germany, France, the UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil.
Figure 2: MEDICAL DEVICE DESIGN AND DEVELOPMENT SERVICES MARKET SHARE BY REGION 2023 (USD Billion)
Europe Medical Device Design and Development Services market accounts for the second-largest market share due to a well-established medical device industry. Europe has captured the second-largest market share in the Medical Device Design and Development Services Market due to several factors. The region benefits from a strong healthcare system, a well-established medical device industry, and a skilled workforce specializing in healthcare innovation. Additionally, Europe's commitment to research and development, collaboration between academia and industry, and adherence to stringent regulatory standards contribute to its prominence in the market.
Furthermore, Europe serves as a hub for medical device innovation, attracting investments and partnerships from global players, thereby solidifying its position as a key player in medical device design and development services. Further, the German Medical Device Design and Development Services market held the largest market share, and the UK Medical Device Design and Development Services market was the fastest growing market in the European region
The Asia-Pacific Medical Device Design and Development Services Market is expected to grow at the fastest CAGR from 2024 to 2032. The Asia Pacific region is experiencing the highest Compound Annual Growth Rate (CAGR) in the Medical Device Design and Development Services Market due to several factors. These include rapid economic growth, increasing healthcare expenditure, and expanding healthcare infrastructure. Moreover, the region benefits from a large pool of skilled professionals, technological advancements, and a growing demand for healthcare services.
Additionally, supportive government initiatives, rising investments in research and development, and the presence of key market players contribute to the accelerated growth of the Medical Device Design and Development Services Market in Asia Pacific. Moreover, China’s Medical Device Design and Development Services market held the largest market share, and the Indian Medical Device Design and Development Services market was the fastest growing market in the Asia-Pacific region.