Regulatory Compliance
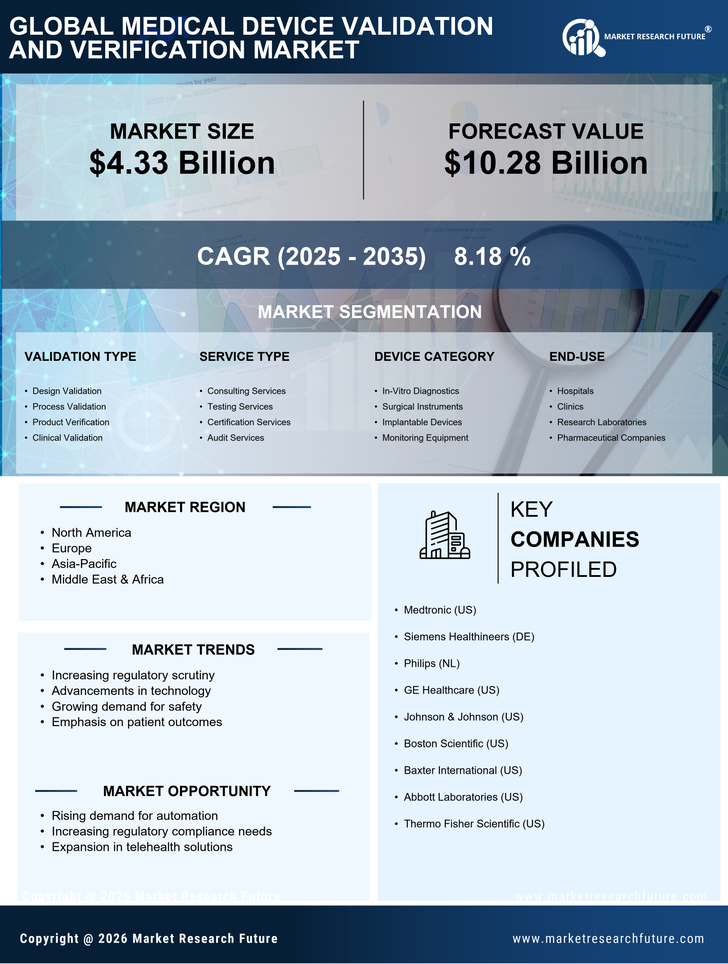
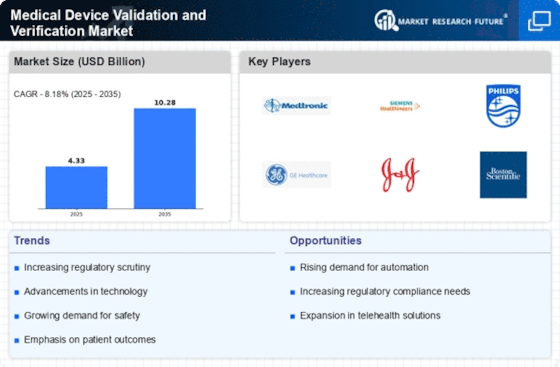
Regulatory compliance remains a pivotal driver in the Medical Device Validation and Verification Market. With stringent regulations imposed by authorities such as the FDA and EMA, manufacturers are compelled to adhere to rigorous validation standards. This compliance is not merely a formality; it is essential for ensuring patient safety and device effectiveness. The market is projected to expand as companies invest in comprehensive validation processes to meet these regulatory requirements. In fact, the market for medical device validation services is expected to reach USD 5 billion by 2026, reflecting the increasing emphasis on compliance and quality assurance in the industry.
Global Health Initiatives
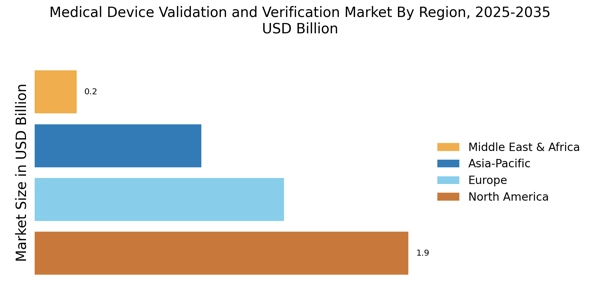
The Medical Device Validation and Verification Industry. As countries strive to enhance their healthcare systems, there is an increasing focus on the development and validation of medical devices that meet international standards. These initiatives often require compliance with rigorous validation processes to ensure that devices are safe and effective for diverse populations. Consequently, the demand for validation services is likely to rise as manufacturers seek to align their products with these global health objectives, thereby expanding the market.
Technological Advancements
The Medical Device Validation and Verification Market is experiencing a surge in technological advancements that enhance the efficiency and accuracy of validation processes. Innovations such as artificial intelligence and machine learning are being integrated into validation protocols, allowing for more precise data analysis and risk assessment. These technologies can potentially reduce the time required for validation, which is crucial in a market where speed to market is essential. Furthermore, the increasing complexity of medical devices necessitates robust validation methods to ensure safety and efficacy. As a result, the demand for advanced validation tools and methodologies is likely to grow, driving the market forward.
Increased Investment in R&D
Investment in research and development is a critical driver for the Medical Device Validation and Verification Market. As companies strive to innovate and develop new medical devices, the need for thorough validation and verification processes becomes paramount. This investment not only facilitates the creation of cutting-edge devices but also ensures that these innovations are safe and effective for patient use. The market is witnessing a trend where organizations are dedicating a larger portion of their budgets to R&D, which in turn drives the demand for validation services. It is anticipated that this trend will continue, further stimulating market growth.
Rising Demand for Quality Assurance
The growing emphasis on quality assurance in the Medical Device Validation and Verification Market is a significant driver of market growth. As healthcare providers and patients alike demand higher standards of safety and efficacy, manufacturers are increasingly prioritizing validation and verification processes. This trend is further fueled by the rising incidence of medical device recalls and safety concerns, which highlight the need for rigorous testing and validation. Consequently, companies are likely to allocate more resources towards ensuring that their devices meet the highest quality standards, thereby propelling the market for validation services and technologies.