Adoption of Advanced Technologies
The Medical Writing Market is witnessing a transformative shift due to the adoption of advanced technologies. Tools such as artificial intelligence and data analytics are increasingly being utilized to streamline the writing process, improve accuracy, and enhance efficiency. In 2025, it is projected that the integration of these technologies will lead to a reduction in turnaround times for medical writing projects, thereby meeting the fast-paced demands of the industry. This technological advancement not only benefits medical writers but also ensures that clients receive high-quality documentation that adheres to regulatory standards.
Emphasis on Quality and Compliance
Quality and compliance are paramount in the Medical Writing Market, particularly as regulatory agencies impose stricter guidelines on documentation. The need for high-quality medical writing is underscored by the increasing complexity of clinical trials and the necessity for precise reporting. In 2025, it is anticipated that regulatory compliance will drive a significant portion of the demand for medical writing services, as companies seek to avoid costly delays in drug approvals. This focus on quality not only enhances the credibility of clinical data but also fosters trust among stakeholders, including regulatory authorities and healthcare professionals.
Rising Need for Clinical Documentation
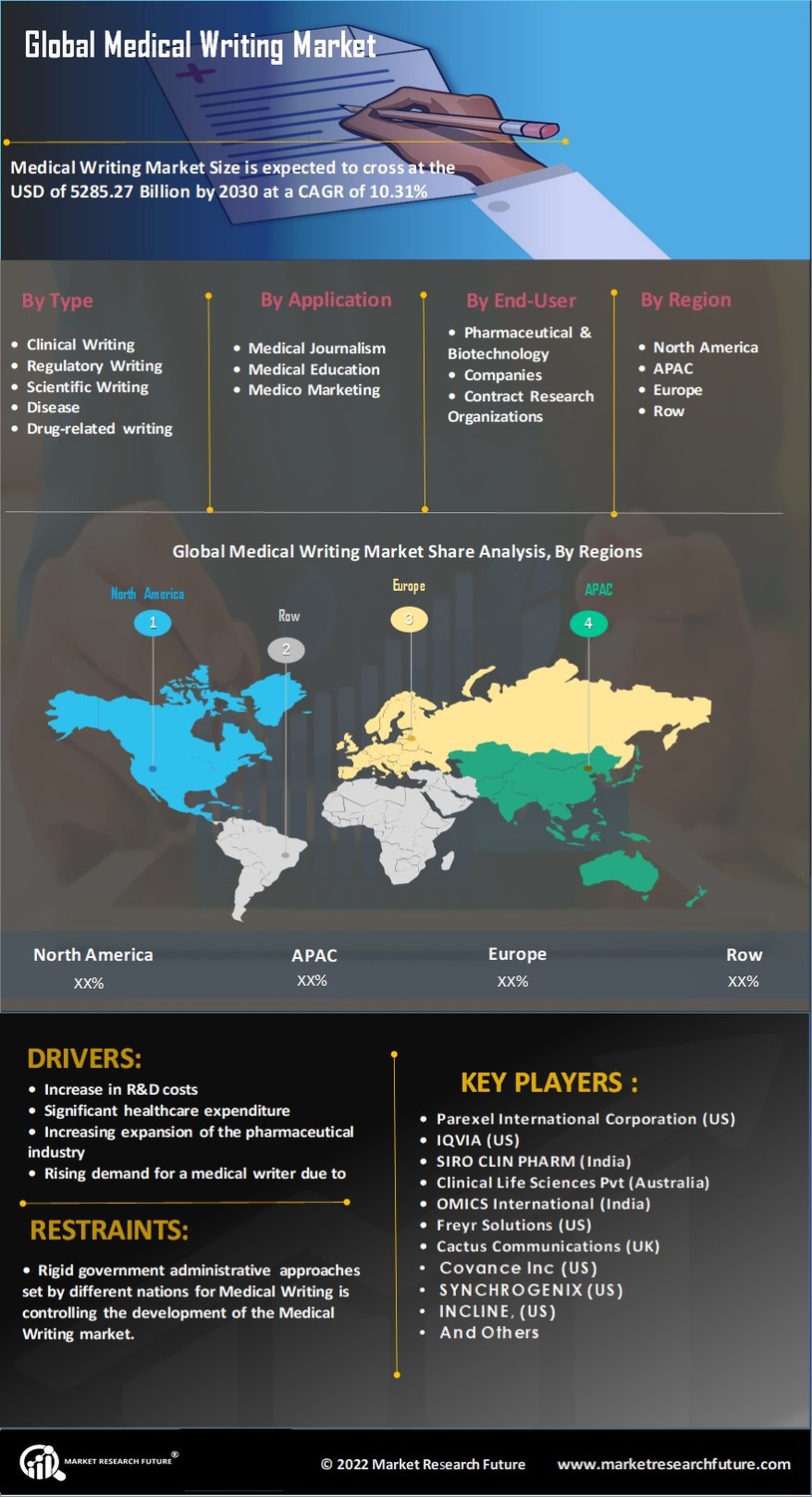
The Medical Writing Market is experiencing a notable increase in the demand for clinical documentation. This surge is primarily driven by the growing number of clinical trials and the need for comprehensive reporting to regulatory bodies. As of 2025, the number of clinical trials has reached unprecedented levels, necessitating skilled medical writers to ensure that documentation meets stringent regulatory standards. The industry is projected to grow at a compound annual growth rate of approximately 7% over the next few years, reflecting the critical role that accurate and thorough clinical documentation plays in the success of drug development and approval processes.
Growing Focus on Patient-Centric Communication
The Medical Writing Market is increasingly prioritizing patient-centric communication as healthcare stakeholders recognize the importance of engaging patients in the clinical process. This trend is reflected in the development of materials that are accessible and understandable to patients, which is essential for informed consent and adherence to treatment protocols. By 2025, the emphasis on patient-centric communication is expected to drive demand for medical writers who can create clear and concise documentation tailored to patient needs. This shift not only enhances patient engagement but also aligns with regulatory expectations for transparency and clarity in medical communications.
Expansion of Pharmaceutical and Biotechnology Sectors
The Medical Writing Market is significantly influenced by the expansion of the pharmaceutical and biotechnology sectors. As these industries continue to evolve, there is an increasing requirement for specialized medical writing services to support drug development, regulatory submissions, and marketing materials. In 2025, the pharmaceutical market is estimated to be valued at over 1.5 trillion USD, which underscores the potential for growth in medical writing services. This expansion creates opportunities for medical writers to engage in diverse projects, ranging from clinical trial protocols to patient information leaflets, thereby enhancing the overall quality of medical communication.















Leave a Comment