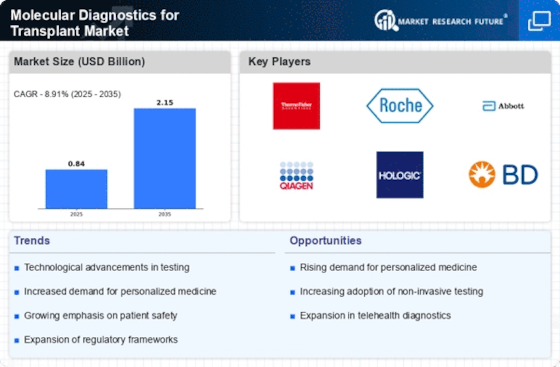
Advancements in Genomic Technologies
Technological innovations in genomic sequencing and analysis are revolutionizing the Molecular Diagnostics for Transplant Market. The advent of next-generation sequencing (NGS) allows for comprehensive genetic profiling of both donors and recipients, enhancing the precision of transplant matching. This capability not only improves the likelihood of transplant success but also reduces the risk of rejection. Market data indicates that the NGS segment is expected to grow at a substantial rate, reflecting the increasing reliance on advanced molecular techniques in transplant diagnostics. Such advancements are likely to foster a more personalized approach to transplantation, aligning with the broader trends in healthcare.
Rising Demand for Personalized Medicine
The shift towards personalized medicine is significantly impacting the Molecular Diagnostics for Transplant Market. As healthcare moves away from a one-size-fits-all approach, there is an increasing emphasis on tailoring treatments to individual patient profiles. Molecular diagnostics play a pivotal role in this paradigm shift by providing critical insights into genetic compatibility and potential responses to immunosuppressive therapies. Market analysis suggests that the demand for personalized diagnostic solutions is on the rise, as healthcare providers seek to optimize transplant outcomes and minimize complications. This trend is likely to continue, further driving innovation and investment in the molecular diagnostics sector.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases, such as diabetes and hypertension, is driving the demand for organ transplants. As these conditions lead to organ failure, the need for effective transplant solutions becomes paramount. The Molecular Diagnostics for Transplant Market is witnessing a surge in demand for diagnostic tools that can accurately assess organ compatibility and monitor transplant success. According to recent data, the prevalence of end-stage renal disease has increased significantly, necessitating more kidney transplants. This trend underscores the importance of molecular diagnostics in ensuring better patient outcomes and optimizing transplant procedures.
Growing Awareness of Transplantation Benefits
There is a notable increase in awareness regarding the benefits of organ transplantation, which is positively influencing the Molecular Diagnostics for Transplant Market. Educational campaigns and advocacy efforts are encouraging more individuals to consider organ donation and transplantation as viable options for treating end-stage organ failure. This heightened awareness is leading to an increase in transplant procedures, thereby driving the demand for molecular diagnostics that can facilitate better donor-recipient matching. As more patients seek transplants, the need for reliable diagnostic tools becomes increasingly critical, further propelling market growth.
Regulatory Support for Diagnostic Innovations
Regulatory bodies are increasingly supporting innovations in the Molecular Diagnostics for Transplant Market, which is fostering a conducive environment for the development of new diagnostic tools. Streamlined approval processes and guidelines for molecular diagnostics are encouraging companies to invest in research and development. This regulatory support is crucial for bringing advanced diagnostic solutions to market more efficiently, ultimately enhancing patient care in transplantation. The presence of favorable regulations is likely to stimulate competition among diagnostic manufacturers, leading to a wider array of options for healthcare providers and patients alike.