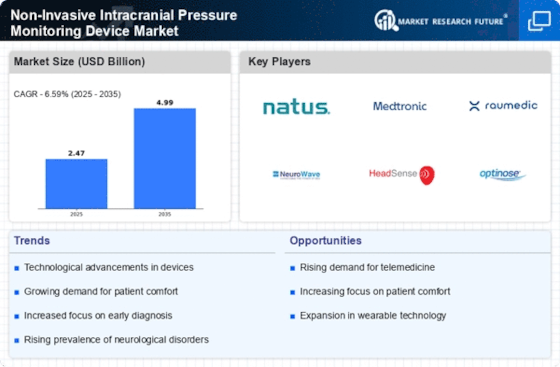
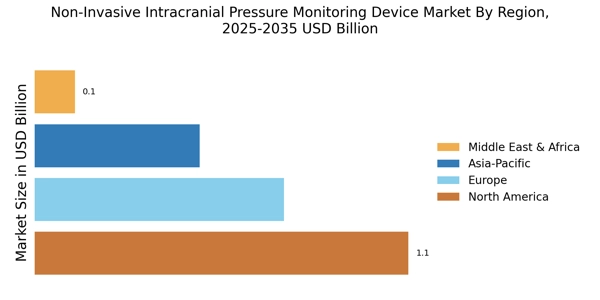
Focus on Minimally Invasive Procedures
The Non-Invasive Intracranial Pressure Monitoring Device Market is benefiting from a growing focus on minimally invasive procedures. Healthcare providers are increasingly recognizing the advantages of non-invasive monitoring techniques, which reduce patient discomfort and recovery time. This shift is particularly relevant in critical care settings, where timely and accurate monitoring is essential. The market is projected to witness a significant increase in demand as more clinicians opt for non-invasive solutions over traditional invasive methods. This trend aligns with broader healthcare goals of improving patient safety and outcomes, thereby driving the Non-Invasive Intracranial Pressure Monitoring Device Market forward.
Rising Incidence of Neurological Disorders
The Non-Invasive Intracranial Pressure Monitoring Device Market is significantly influenced by the rising incidence of neurological disorders. Conditions such as traumatic brain injury, stroke, and hydrocephalus are becoming more prevalent, necessitating effective monitoring solutions. According to recent estimates, the global burden of neurological disorders is expected to increase, leading to a higher demand for non-invasive monitoring devices. This trend is particularly evident in aging populations, where the risk of such disorders escalates. As healthcare systems strive to manage these conditions more effectively, the Non-Invasive Intracranial Pressure Monitoring Device Market is poised for growth, with an increasing number of healthcare facilities adopting these technologies.
Growing Awareness and Education on Brain Health
The Non-Invasive Intracranial Pressure Monitoring Device Market is witnessing growth due to increasing awareness and education regarding brain health. Public health campaigns and educational initiatives are emphasizing the importance of early detection and monitoring of neurological conditions. As patients and healthcare providers become more informed about the risks associated with elevated intracranial pressure, the demand for non-invasive monitoring solutions is likely to rise. This heightened awareness is fostering a more proactive approach to brain health, which could lead to a significant uptick in the adoption of non-invasive devices. Thus, the Non-Invasive Intracranial Pressure Monitoring Device Market is expected to expand as awareness continues to grow.
Technological Advancements in Monitoring Devices
The Non-Invasive Intracranial Pressure Monitoring Device Market is experiencing a surge in technological advancements. Innovations such as advanced sensors and imaging techniques are enhancing the accuracy and reliability of non-invasive monitoring. For instance, devices utilizing transcranial Doppler ultrasound and near-infrared spectroscopy are gaining traction. These technologies not only improve patient outcomes but also reduce the need for invasive procedures, which can be risky. The market is projected to grow at a compound annual growth rate of approximately 8% over the next five years, driven by these advancements. As healthcare providers increasingly adopt these technologies, the Non-Invasive Intracranial Pressure Monitoring Device Market is likely to expand, catering to a broader range of neurological conditions.
Increased Investment in Healthcare Infrastructure
The Non-Invasive Intracranial Pressure Monitoring Device Market is also being propelled by increased investment in healthcare infrastructure. Governments and private entities are allocating substantial resources to enhance healthcare facilities, particularly in developing regions. This investment is aimed at improving access to advanced medical technologies, including non-invasive monitoring devices. As healthcare systems evolve, the demand for innovative solutions that can provide real-time data on intracranial pressure is likely to rise. Consequently, the Non-Invasive Intracranial Pressure Monitoring Device Market stands to benefit from this trend, as more healthcare providers seek to integrate these devices into their practice.