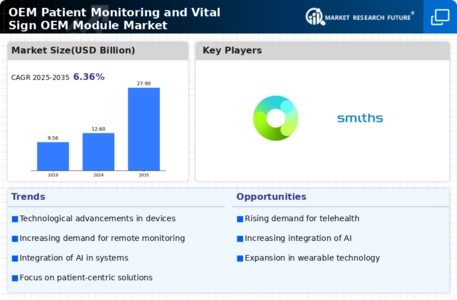
Top Industry Leaders in the OEM Patient Monitoring Vital Sign OEM Module Market
 Latest Oem Patient Monitoring Vital Sign Oem Module Companies Update
Latest Oem Patient Monitoring Vital Sign Oem Module Companies Update
-
June 2023: Leading health technology companies Royal Philips and Masimo have announced FDA authorization for SedLine® Brain Function Monitoring, Regional Oximetry (O3®), and CO₂ readings to be enabled in Philips Patient Monitors, IntelliVue MX750 and MX850. With the newest development in Masimo and Philips' ongoing partnership, physicians will be better equipped to act quickly and intelligently without requiring extra monitoring tools. Clinicians can more easily assess and monitor cerebral oxygenation (brain saturation), anesthesia sedation, and patient respiratory performance with the integration of SedLine, O3®, and CO₂ advanced Masimo figures into Philips high acuity IntelliVue® MX series multi-parameter monitors. Monitors can also exchange data with one another.
-
May 2023: A series of final agreements have been reached by Medtronic plc, an Irish healthcare technology company, to purchase South Korea's EOFlow Co., the company behind the EOPatch wearable temporary insulin delivery device. The entire consideration for purchasing the shares in EOFlow would be KRW 971 billion, about $738 million, at current exchange rates, if all the public shares participate in the tender offer. Closing of the deal is anticipated for the second half of 2023. Integrating EOFlow with Medtronic's next-generation continuous glucose monitor (CGM) and Meal Detection Technology algorithm is anticipated to enhance the company's capacity to cater to the requirements of more persons with diabetes. The EOPatch device has been approved for commercialization. It uses a patented microfluidic technology that reduces the danger of insulin occlusion while delivering insulin with great accuracy and consistency.
List of OEM Patient Monitoring Vital Sign OEM Module Key companies in the market
- Sentec AG (Switzerland)
- Medtronic Plc (Ireland)
- Nonin Medical Inc. (US)
- Masimo Corporation (US)
- Ronseda Electronics Co., Ltd (China)
- Triton Electronic Systems Ltd (Russia)
- Halma Plc (UK)
- Zug Medical System SAS (France)
- Fresenius Kabi AG (Germany)
- Smiths Group plc (UK)