Research Methodology on Orphan Drugs Market
1. Introduction
This research methodology outlines the methods and approaches applied in the study of the orphan drugs market, as highlighted by Market Research Future in its report. This methodology explains the components of the research design, data collection procedure, sampling technique, and data analysis and interpretation. The analysis of the global orphan drugs market involves a thorough assessment of the key stakeholders, industry trends, current and evolving market dynamics, and drivers and restraints that are expected to directly and/or indirectly impact the market’s growth during the assessment period (2023-2030).
2. Research Design
To collect necessary information, a qualitative and quantitative research design is employed in the market study. The objective of the market study is to size, forecast and analyze the global orphan drugs market for 2023-2030. For this purpose, Market Research Future conducted primary and secondary research on the global orphan drugs market, applying several methodologies such as surveys, technological advancements, etc.
3. Data Collection Procedure
Primary research is conducted through face-to-face, telephonic, and online interviews with industry participants such as CEOs, CFOs, market analysts and regional managers. Data from industry experts and opinion leaders are used to develop a fresh perspective on the market. Industry experts, articles, press releases, white papers, and webinars are used to gather secondary data about the orphan drugs market.
The primary research investors were able to validate the accuracy and complement the secondary research findings. Additionally, the inputs and opinions collected from the end-users, distributors, consultations, and research experts provide comprehensive insights into the global market.
4. Sampling Methodology
The market study is conducted in the following six segments: type, mode of administration, therapeutic application, route of delivery, indications, and region. Under the type segment, monoclonal antibodies, proteins, small molecules and others are studied; and according to the mode of administration segment, oral, parenteral, and others are studied. Additionally, the therapeutic application segment is divided into oncology, rare genetic diseases, rare metabolic diseases, and others; the route of the delivery segment is divided into chronic and acute; the indications segment is divided into rare endocrine disorders, gastroenterological diseases, infections, musculoskeletal disorders, and other indications.
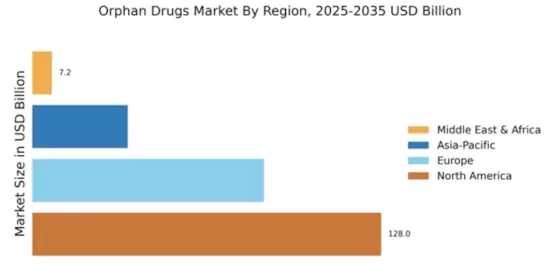
The market is studied in four regions: North America, Europe, Asia-Pacific, and the rest of the world.
5. Data Analysis and Interpretation
In the market study, Market Research Future employed Porter’s five forces analysis, market segmentation, market attractiveness analysis, and value chain analysis. These tools and techniques are used to analyze the orphan drugs market. Additionally, spreadsheets, databases, and MS Office tools are used to analyze the collected data and generate useful insights.
To draw conclusions and make recommendations, Market Research Future conducted interviews with industry players and developed an in-depth analysis of the overall orphan drugs market. The market is segmented and analyzed based on the primary and secondary segmentation variables to identify various trends and insights, which in turn helped shape the market forecast. The market report is further validated using a triangulation method.
Overall, the data collection and analysis procedure is conducted using the most up-to-date data sources and primary and secondary research inputs from industry experts. The resulting market report offered comprehensive insights into the overall orphan drugs market.