Market Analysis
In-depth Analysis of Orphan Drugs Market Industry Landscape
In recent times, there has been remarkable progress in the development of new technologies, including DNA recombinant, hybridoma, and gene mapping. This advancement is primarily attributed to the increasing prevalence of rare diseases and the growing acceptance of DNA recombinant technology among healthcare professionals. The adoption of these technologies has been swift, facilitated by their low marketing costs and the allure of extensive exclusivity.
One significant factor contributing to this technological surge is the rising approval rate for advanced gene mapping devices by the US Food and Drug Administration (FDA). The FDA plays a crucial role in evaluating and approving medical technologies, and its positive stance on advanced gene mapping devices is expected to significantly boost market growth.
For many years, scientists have been diligently researching gene therapies aimed at treating rare disorders. The breakthrough came in 2017 when the US FDA approved the first gene therapy designed specifically for patients with rare disorders. This marks a significant milestone in the medical field, as it opens new avenues for treating conditions that were previously deemed untreatable. Gene therapy, as a novel treatment modality, presents unique technical challenges, particularly when dealing with rare diseases.
A notable example highlighting the potential of gene therapy is the approval of two therapies developed by Sangamo Therapeutics for treating hemophilia A and B. These therapies received special regulatory designations from the US FDA, underscoring their importance in addressing rare disorders.
In conclusion, the ongoing development of new technologies tailored for orphan drugs, such as DNA recombinant, hybridoma, and gene mapping, is poised to provide substantial opportunities for market growth in the coming years. The increasing prevalence of rare diseases, coupled with the endorsement of DNA recombinant technology within the healthcare community, sets the stage for significant advancements. Moreover, the FDA's positive outlook on advanced gene therapies further solidifies the potential for breakthroughs in treating rare diseases. The continuous evolution of these technologies reflects a promising outlook for the landscape of rare disease treatment.


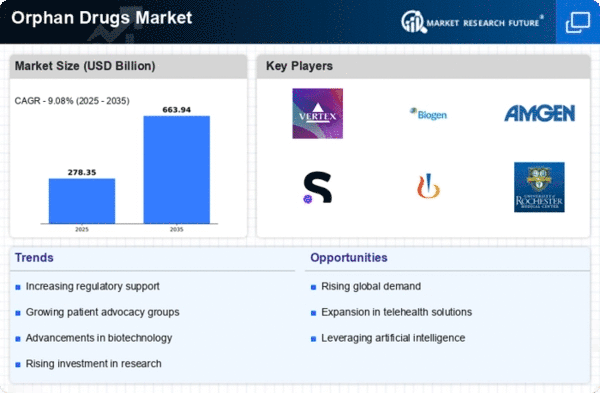















Leave a Comment