Market Share
Orphan Drugs Market Share Analysis
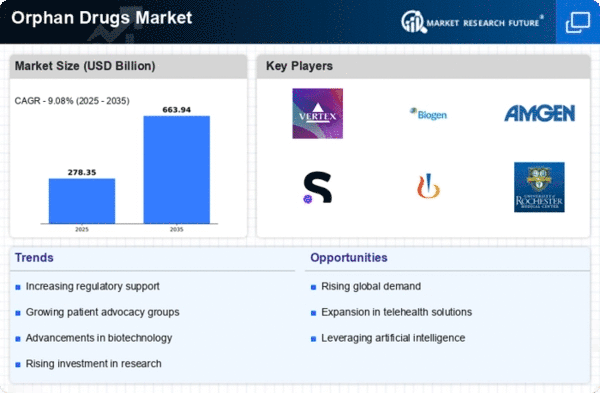
As the prevalence of rare diseases continues to rise, pharmaceutical and biotech companies are actively engaged in researching and developing new medications to address these uncommon medical conditions. Rare disorders are defined as those affecting less than 200,000 individuals in the United States or fewer than 5 per 10,000 individuals in the European Union. To bridge the gap in drug development for rare diseases, regulatory agencies in the United States and the European Union have taken proactive measures to encourage the creation of new medicines.
Researchers have made significant strides in understanding how to diagnose, treat, and prevent various rare diseases. In the United States, the Food and Drug Administration (FDA) has committed support for rare disease treatment research through funding allocated to the Orphan Products Grants Program, a component of the Orphan Drug Act. This program has disbursed over USD 300 million, supporting more than 530 new clinical studies that have led to the marketing approval of 50 products.
Several organizations, such as the Medical Research Charities Group, also contribute to funding orphan drug research. Specific rare disease research groups, like those focused on conditions such as epidermolysis bullosa and retinitis pigmentosa, receive funding from international medical research charities such as DEBRA International and the Wellcome Trust.
The National Institutes of Health (NIH) play a crucial role in advancing rare disease research by funding researchers to develop new therapies that enhance overall health. Within the NIH, many of the 27 Institutes and Centers allocate funds for medical research on rare diseases. The National Center for Advancing Translational Sciences (NCATS), one of these centers, prioritizes accelerating the delivery of new cures and treatments to patients. NCATS supports research through collaborative projects that explore common themes and causes of various diseases, aiming to expedite the development of treatments benefiting both rare and common diseases.
The Office of Orphan Products Development (OOPD) is instrumental in providing funding to drug companies to develop treatments specifically tailored for rare diseases. This financial support contributes to the overall progress in the field of rare disease research and ensures that novel treatments are developed to improve the lives of those affected.

















Leave a Comment