Increased Awareness and Education
Increased awareness and education regarding Paroxysmal Nocturnal Hemoglobinuria Market are pivotal drivers for the Paroxysmal Nocturnal Hemoglobinuria Market. As healthcare providers and patients become more informed about the symptoms and complications associated with PNH, the likelihood of early diagnosis and treatment increases. Educational campaigns and initiatives by patient advocacy groups are playing a crucial role in disseminating information about PNH, which may lead to a higher rate of diagnosis. This heightened awareness is likely to result in an uptick in demand for diagnostic tests and treatment options, thereby propelling market growth. Additionally, as more healthcare professionals recognize the importance of timely intervention in PNH, the overall management of the disease is expected to improve, further driving the Paroxysmal Nocturnal Hemoglobinuria Market.
Regulatory Support and Incentives
Regulatory support and incentives for the development of therapies targeting Paroxysmal Nocturnal Hemoglobinuria Market are likely to play a crucial role in shaping the Paroxysmal Nocturnal Hemoglobinuria Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for orphan drugs, which include treatments for rare diseases like PNH. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, as they can benefit from incentives such as tax credits and market exclusivity. The potential for faster access to the market for innovative therapies is expected to stimulate competition and drive advancements in treatment options for PNH. As a result, regulatory support is anticipated to be a key driver of growth within the Paroxysmal Nocturnal Hemoglobinuria Market.
Advancements in Treatment Modalities
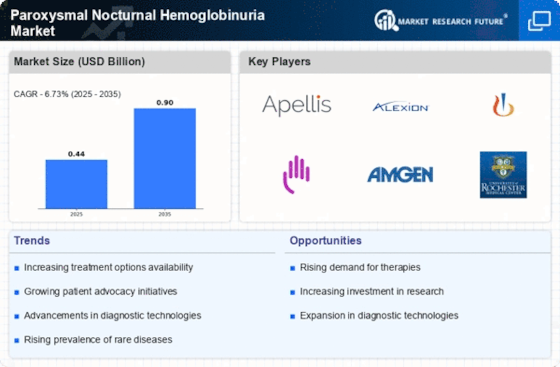
Recent advancements in treatment modalities for Paroxysmal Nocturnal Hemoglobinuria Market are significantly influencing the Paroxysmal Nocturnal Hemoglobinuria Market. The introduction of complement inhibitors, such as eculizumab and ravulizumab, has transformed the therapeutic landscape for PNH patients. These treatments have demonstrated efficacy in reducing hemolysis and improving quality of life, which is likely to attract more patients seeking effective management options. Market data indicates that the global market for PNH therapies is projected to reach several billion dollars by the end of the decade, reflecting the growing recognition of the need for specialized treatments. Furthermore, ongoing clinical trials and research initiatives are expected to yield new therapies, potentially expanding the treatment options available for PNH. This continuous innovation in treatment modalities is a key driver for the Paroxysmal Nocturnal Hemoglobinuria Market.
Collaborative Research and Development Efforts
Collaborative research and development efforts among pharmaceutical companies, academic institutions, and healthcare organizations are emerging as a significant driver for the Paroxysmal Nocturnal Hemoglobinuria Market. These collaborations are fostering innovation and accelerating the development of new therapies for PNH. By pooling resources and expertise, stakeholders are better positioned to conduct comprehensive clinical trials and research studies that can lead to breakthroughs in treatment. The increasing number of partnerships and alliances in the field of hematology is indicative of a collective commitment to addressing the unmet needs of PNH patients. This collaborative approach not only enhances the understanding of PNH but also facilitates the introduction of novel therapies into the market, thereby contributing to the growth of the Paroxysmal Nocturnal Hemoglobinuria Market.
Rising Incidence of Paroxysmal Nocturnal Hemoglobinuria
The increasing incidence of Paroxysmal Nocturnal Hemoglobinuria Market (PNH) is a notable driver for the Paroxysmal Nocturnal Hemoglobinuria Market. Recent estimates suggest that the prevalence of PNH is approximately 5 cases per million people, indicating a growing patient population. This rise in incidence is likely to lead to heightened demand for effective treatment options, thereby stimulating market growth. As awareness of PNH improves among healthcare professionals and patients, more individuals are being diagnosed, which could further contribute to the expansion of the market. The need for innovative therapies and management strategies for PNH is becoming increasingly apparent, as the condition can lead to severe complications if left untreated. Consequently, the rising incidence of PNH is expected to drive investments in research and development within the Paroxysmal Nocturnal Hemoglobinuria Market.
.png)