Increased Research Funding
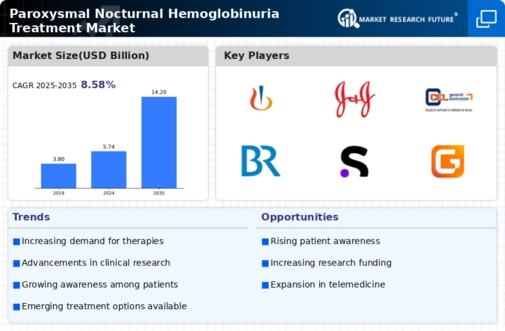
Increased research funding for Paroxysmal Nocturnal Hemoglobinuria is a pivotal driver for the Paroxysmal Nocturnal Hemoglobinuria Treatment Market. Government and private organizations are recognizing the need for more effective treatments and are allocating resources to support research initiatives. This influx of funding is facilitating the exploration of novel therapeutic approaches and the development of clinical trials aimed at improving patient care. As a result, the number of investigational drugs entering the market is expected to rise, potentially leading to breakthroughs in PNH treatment. Furthermore, enhanced research efforts may also contribute to a better understanding of the disease's pathophysiology, which could inform future therapeutic strategies. Thus, the increase in research funding is likely to play a crucial role in shaping the future of the Paroxysmal Nocturnal Hemoglobinuria Treatment Market.
Advancements in Treatment Options
Advancements in treatment options for Paroxysmal Nocturnal Hemoglobinuria are significantly influencing the Paroxysmal Nocturnal Hemoglobinuria Treatment Market. The introduction of novel therapies, such as complement inhibitors, has transformed the management of PNH, providing patients with more effective and targeted treatment alternatives. For instance, therapies like eculizumab and ravulizumab have shown promising results in clinical trials, leading to their approval for use in PNH patients. These advancements not only improve patient outcomes but also attract investment from pharmaceutical companies eager to capitalize on the growing market. As new therapies continue to emerge, the competitive landscape of the Paroxysmal Nocturnal Hemoglobinuria Treatment Market is likely to evolve, fostering innovation and enhancing treatment accessibility for patients.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups is fostering a more informed patient population, which is driving the Paroxysmal Nocturnal Hemoglobinuria Treatment Market. These organizations play a vital role in raising awareness about PNH, educating patients about their treatment options, and advocating for better access to therapies. As patients become more knowledgeable about their condition, they are more likely to seek treatment, thereby increasing demand within the market. Additionally, these groups often collaborate with healthcare providers and pharmaceutical companies to promote research and development efforts, further enhancing the treatment landscape. The growing presence of patient advocacy groups is likely to empower individuals with PNH, leading to improved health outcomes and a more robust market for PNH treatments.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant driver of the Paroxysmal Nocturnal Hemoglobinuria Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for treatments targeting rare diseases like PNH. Initiatives such as orphan drug designations and fast-track approvals are encouraging pharmaceutical companies to invest in the development of new therapies. This supportive regulatory environment not only accelerates the availability of novel treatments but also enhances market competition, as more companies enter the field. As a result, patients are likely to benefit from a wider array of treatment options, ultimately improving their quality of life. The regulatory support for innovative therapies is thus a crucial factor influencing the dynamics of the Paroxysmal Nocturnal Hemoglobinuria Treatment Market.
Rising Incidence of Paroxysmal Nocturnal Hemoglobinuria
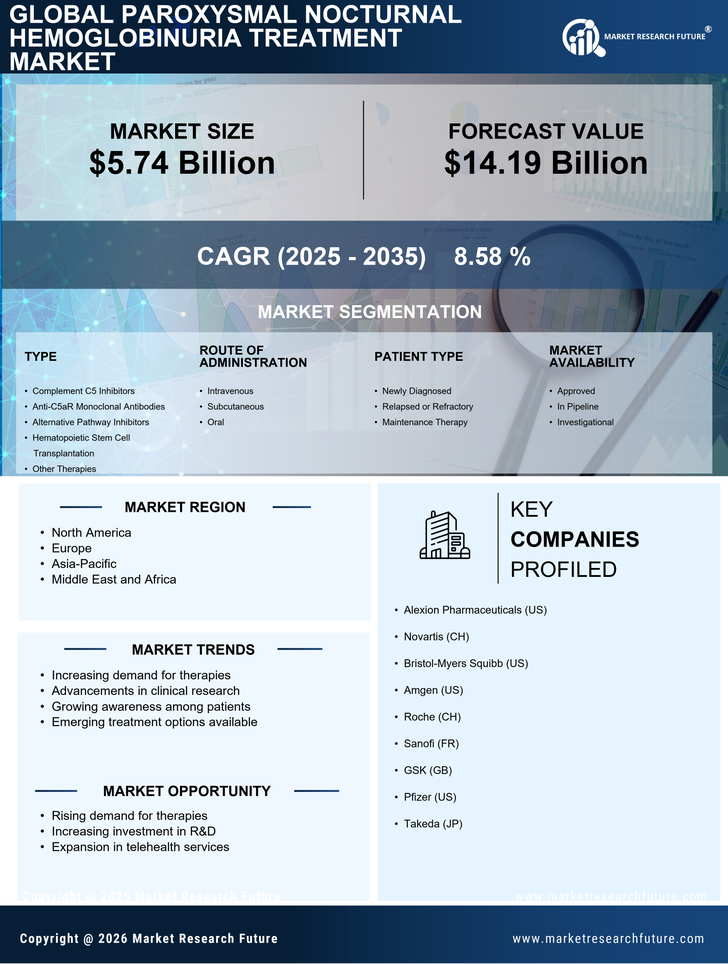
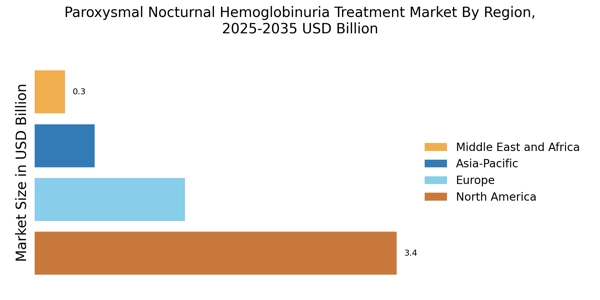
The increasing incidence of Paroxysmal Nocturnal Hemoglobinuria (PNH) is a notable driver for the Paroxysmal Nocturnal Hemoglobinuria Treatment Market. Recent estimates suggest that the prevalence of PNH is approximately 1 to 5 cases per million people, indicating a growing patient population that requires effective treatment options. As awareness of this rare disease expands, more individuals are being diagnosed, which in turn fuels demand for innovative therapies. The rise in incidence is likely to stimulate research and development efforts, leading to the introduction of new treatment modalities. This trend is expected to enhance the overall market landscape, as pharmaceutical companies focus on addressing the unmet needs of PNH patients. Consequently, the increasing incidence of PNH is a critical factor driving growth in the Paroxysmal Nocturnal Hemoglobinuria Treatment Market.