Advancements in Immunotherapy
Recent advancements in immunotherapy are significantly influencing the PD-1 Resistant Head and Neck Cancer Market. The exploration of novel immune checkpoint inhibitors and combination therapies has shown promise in overcoming resistance mechanisms associated with PD-1 therapies. For instance, studies have demonstrated that combining PD-1 inhibitors with other immunotherapeutic agents can enhance treatment efficacy, potentially leading to improved patient outcomes. The market is witnessing a surge in research and development activities aimed at identifying effective combinations that can address PD-1 resistance. As these innovative therapies progress through clinical trials, they are expected to reshape treatment paradigms and expand the therapeutic landscape for head and neck cancer patients, thereby driving market growth.
Patient Awareness and Advocacy
Patient awareness and advocacy are playing an increasingly influential role in the PD-1 Resistant Head and Neck Cancer Market. As patients become more informed about their treatment options, there is a growing demand for effective therapies that address PD-1 resistance. Advocacy groups are actively promoting awareness of head and neck cancers and the challenges associated with treatment resistance. This heightened awareness is leading to increased patient engagement in clinical trials and a push for more research into alternative therapies. Furthermore, as patients advocate for their needs, pharmaceutical companies are likely to respond by prioritizing the development of innovative solutions. This trend not only empowers patients but also drives market growth by fostering a more responsive and patient-centered approach to treatment.
Growing Investment in Cancer Research
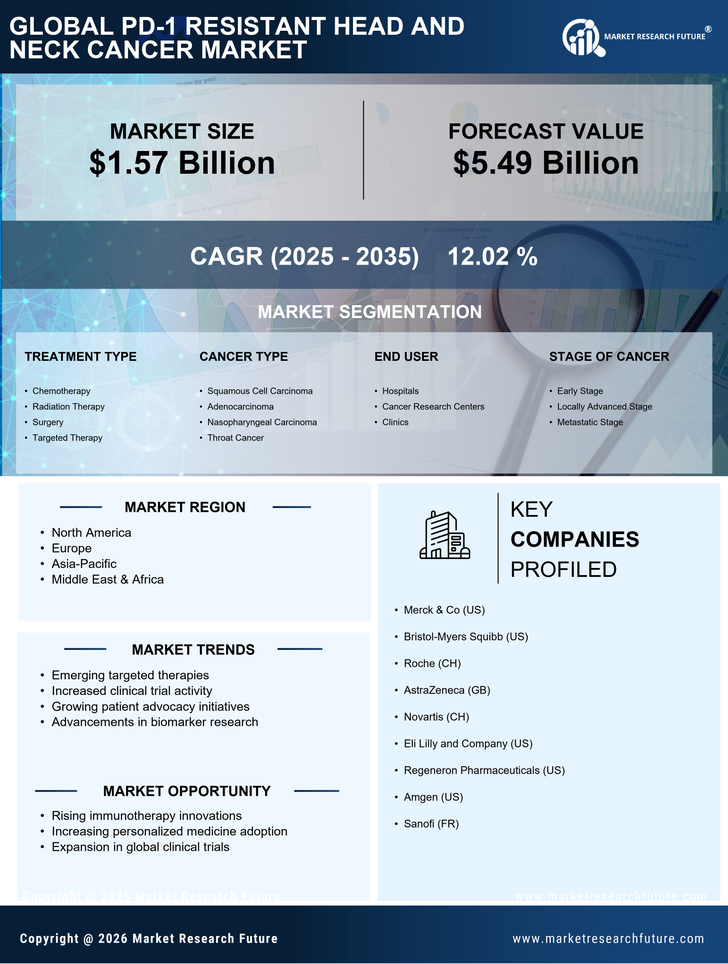
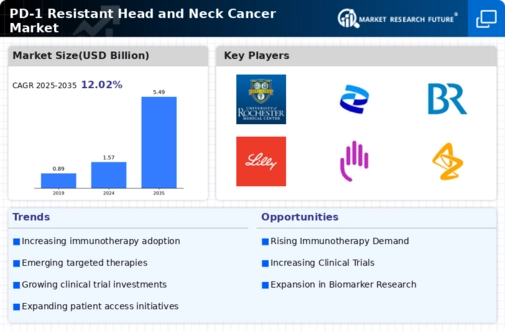
The growing investment in cancer research is a crucial driver for the PD-1 Resistant Head and Neck Cancer Market. Increased funding from both public and private sectors has facilitated extensive research into the mechanisms of resistance to PD-1 therapies. This financial support has led to the identification of novel biomarkers and therapeutic targets, which are essential for developing effective treatment strategies. According to recent reports, funding for cancer research has seen a significant uptick, with billions allocated annually to explore innovative solutions for resistant cancers. This influx of resources is likely to accelerate the pace of discovery and development of new therapies, ultimately benefiting patients with PD-1 resistant head and neck cancers.
Rising Incidence of Head and Neck Cancer
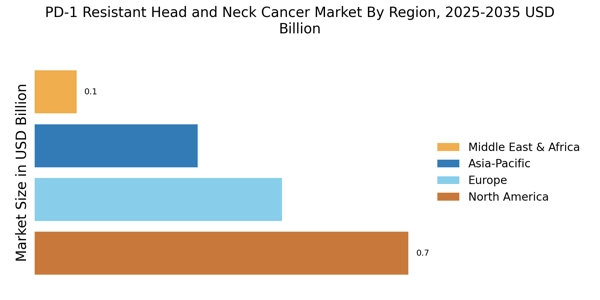
The increasing incidence of head and neck cancer is a pivotal driver for the PD-1 Resistant Head and Neck Cancer Market. Recent statistics indicate that head and neck cancers account for a substantial percentage of all cancer cases, with a notable rise in diagnoses attributed to factors such as tobacco use and human papillomavirus (HPV) infection. This trend necessitates the development of innovative treatment options, particularly for patients who exhibit resistance to PD-1 inhibitors. As the patient population grows, the demand for effective therapies targeting PD-1 resistant tumors is likely to escalate, thereby propelling market growth. Furthermore, the need for tailored treatment strategies to address the unique challenges posed by resistant cancers underscores the urgency for advancements in this sector.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a vital driver in the PD-1 Resistant Head and Neck Cancer Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for breakthrough therapies that address unmet medical needs. This trend is particularly relevant for treatments targeting PD-1 resistant cancers, as the urgency for effective solutions grows. Initiatives such as accelerated approval pathways and priority review designations are being implemented to facilitate the timely introduction of novel therapies into the market. As a result, companies are more inclined to invest in the development of innovative treatments, knowing that regulatory frameworks are evolving to support their efforts. This dynamic is expected to enhance the availability of effective options for patients facing PD-1 resistance.