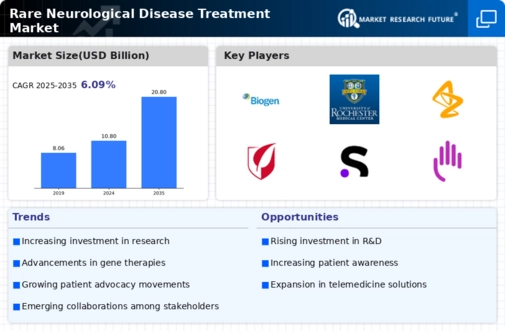
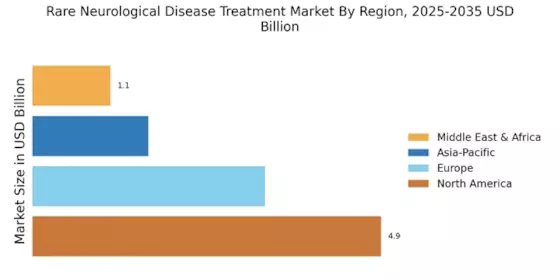
The Rare Neurological Disease Treatment Market is characterized by a complex interplay of innovation, strategic partnerships, and a growing emphasis on personalized medicine.
As of October key players such as Biogen (US), Novartis (CH), and Roche (CH) are actively shaping the competitive landscape through their distinct operational focuses. Biogen (US) continues to prioritize research and development, particularly in gene therapies, which are seen as pivotal in addressing rare neurological disease disorders. Meanwhile, Novartis (CH) has adopted a strategy of regional expansion, particularly in emerging markets, to enhance access to its innovative treatments. Roche (CH), on the other hand, is leveraging its robust pipeline of biologics and small molecules, indicating a strong commitment to innovation in this niche market. Collectively, these strategies not only enhance their market positions but also contribute to a dynamic competitive environment that is increasingly focused on patient-centric solutions.
In terms of business tactics, companies are increasingly localizing manufacturing and optimizing supply chains to enhance efficiency and responsiveness to market demands. The competitive structure of the Rare Neurological Disease Treatment Market appears moderately fragmented, with several players vying for market share. However, the influence of major companies like Biogen (US) and Novartis (CH) is substantial, as their strategic initiatives often set industry standards and drive innovation across the sector.
In August 2025, Biogen (US) announced a groundbreaking partnership with a leading biotech firm to co-develop a novel gene therapy aimed at treating a rare form of amyotrophic lateral sclerosis (ALS). This collaboration is significant as it not only expands Biogen's therapeutic portfolio but also underscores the growing trend of partnerships in the biotech space, which can accelerate the development of critical therapies. The strategic importance of this move lies in its potential to enhance patient outcomes and solidify Biogen's leadership in the rare neurological disease segment.
In September 2025, Novartis (CH) launched a new initiative aimed at increasing access to its treatments in underserved regions, particularly in Asia and Africa. This initiative is noteworthy as it reflects a broader industry trend towards addressing health disparities and ensuring that innovative therapies reach a wider patient population. By focusing on accessibility, Novartis is likely to strengthen its market presence while also contributing to global health equity.
In October 2025, Roche (CH) unveiled a new digital platform designed to facilitate real-time patient monitoring and data collection for its neurological therapies. This strategic move highlights the increasing integration of digital technologies in healthcare, particularly in the management of rare diseases. By harnessing data analytics and AI, Roche aims to enhance treatment efficacy and patient engagement, positioning itself at the forefront of digital transformation in the pharmaceutical industry.
As of October 2025, the competitive trends in the Rare Neurological Disease Treatment Market are increasingly defined by digitalization, sustainability, and the integration of artificial intelligence. Strategic alliances are becoming more prevalent, as companies recognize the value of collaboration in driving innovation and improving patient outcomes. Looking ahead, it appears that competitive differentiation will evolve from traditional price-based competition to a focus on innovation, technological advancements, and the reliability of supply chains. This shift may ultimately lead to a more sustainable and patient-centric approach in the treatment of rare neurological diseases.