Global Trade Regulations
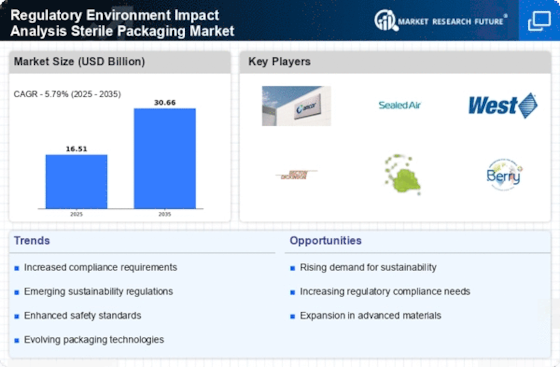
The Regulatory Environment Impact Analysis Sterile Packaging Industry. As international trade continues to expand, compliance with various regional regulations becomes essential for manufacturers. Different countries have distinct requirements for sterile packaging, which can complicate market entry for companies. This complexity may drive businesses to invest in regulatory expertise and adapt their packaging solutions accordingly. The market could experience growth as companies navigate these trade regulations, ensuring that their products meet the necessary standards for different regions, thereby enhancing their competitiveness in the global marketplace.
Sustainability Initiatives
Sustainability initiatives are becoming increasingly pivotal in the Regulatory Environment Impact Analysis Sterile Packaging Market. With growing environmental concerns, regulatory frameworks are evolving to promote eco-friendly packaging solutions. This shift is compelling manufacturers to explore biodegradable and recyclable materials, aligning with regulations that aim to reduce plastic waste. The market is projected to see a rise in demand for sustainable packaging, with estimates suggesting that eco-friendly materials could account for a significant share of the market by 2027. Companies that proactively adapt to these sustainability regulations may gain a competitive edge, as consumers increasingly favor environmentally responsible products.
Technological Advancements
Technological advancements are significantly influencing the Regulatory Environment Impact Analysis Sterile Packaging Market. Innovations in materials science and packaging technology are enabling the development of more effective sterile packaging solutions. For example, the integration of smart packaging technologies, such as RFID and sensors, is enhancing traceability and monitoring of sterile products. These advancements not only comply with regulatory requirements but also improve supply chain efficiency. The market is expected to grow as companies invest in these technologies to meet stringent regulations while ensuring product safety and quality. This trend indicates a potential shift towards more intelligent and responsive packaging systems.
Evolving Regulatory Standards
The Regulatory Environment Impact Analysis Sterile Packaging Market is currently shaped by evolving regulatory standards that aim to enhance safety and efficacy in packaging. Regulatory bodies, such as the FDA and EMA, have established stringent guidelines for sterile packaging to mitigate contamination risks. These regulations necessitate compliance from manufacturers, thereby driving innovation in packaging materials and processes. For instance, the introduction of ISO 11607 standards has prompted companies to adopt advanced sterilization techniques and robust testing protocols. As a result, the market is witnessing a shift towards more reliable and compliant packaging solutions, which could potentially increase operational costs but also enhance product integrity and consumer trust.
Increased Focus on Patient Safety
An increased focus on patient safety is driving changes in the Regulatory Environment Impact Analysis Sterile Packaging Market. Regulatory agencies are emphasizing the importance of packaging integrity to prevent contamination and ensure the safety of medical products. This heightened awareness is leading to stricter regulations and guidelines that manufacturers must adhere to. As a result, companies are investing in advanced testing methods and quality assurance processes to comply with these regulations. The market is likely to expand as organizations prioritize patient safety, which may lead to higher demand for reliable and effective sterile packaging solutions.
.png)