Market Analysis
In-depth Analysis of Retinal Biologics Market Industry Landscape
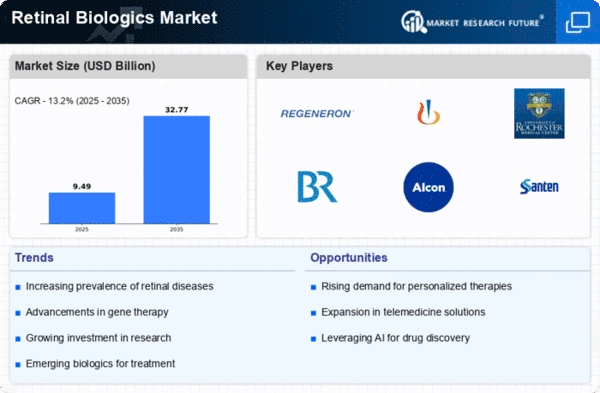
Retinal pharmaceutical biologics market dynamics reveal a focused response to intricate challenges posed by retinal diseases, emphasizing innovative treatment strategies and progressions in biologic therapies. Diseases of the retina such as age-related macular degeneration (AMD), diabetic retinopathy and macular edema impair vision and lead to blindness. The presence of these factors is among those that shaped the market for pharmaceuticals targeting retinal disorders including the increasing prevalence of retinal disorders, need for targeted and efficacious therapies, and ongoing advancements in biotechnology.
The primary driver behind the above market dynamics is an increase in the number of retinal diseases affecting aging populations. The incidence rates of conditions such as AMD and Diabetic Retinopathy continue to rise worldwide necessitating treatments that manage symptoms but also address underlying pathophysiology. Pharmaceutical companies focus on developing biologics against discrete molecular pathways involved in retinal disease rather than traditional interventions.
The dynamics of pharmaceutical retinal biologics market is significantly influenced by advances in biotechnology and understanding of its pathophysiology. Biologics like monoclonal antibodies, fusion proteins target specific molecules or cells associated with different types of problems related to retina. These new treatments aim at modulating immune response, inhibiting abnormal angiogenesis or within retina promoting tissue repair. In what way does the market respond? – it offers various types of retinal bio-therapies targeting unique molecular markers designed for patients suffering from different kinds of eye disorders.
Moreover, research efforts and clinical trials continue investigating novel applications and emerging areas where new drugs can be used as well as assessing other aspects pertaining to new formulations of already existing drugs. As scientific knowledge expands, pharmaceutical companies seek to introduce innovative biological medications into health care industry thus providing more options for doctors and their patients. These clinical trials play an essential role when it comes to assessing safety profiles alongside efficacy levels tied to these products thereby changing how treatment protocols are developed and how care is given.
Regulatory considerations play a major role in shaping the market dynamics of pharmaceutical retinal biologics. Regulators require rigorous standardization of approval and marketing for these types of therapies thus ensuring their safety, efficacy and quality. The nature of retinal disorders necessitates thorough evaluations that focus both on short-term efficacy and long-term safety. Pharmaceutical companies navigate through these regulatory pathways with innovative retinal biologics contributing to the overall trustworthiness and credibility of the treatments available for patients with retinal diseases.
Another influential factor on the market dynamics is healthcare industry’s shift towards personalized medicine and patient-centric care. Clinical heterogeneity in retinal disorders often exist implying that treatment response may vary among individuals. In response, the market seeks to develop personalized bio-therapies that take into account genetics, biomarkers or other patient-specific traits. This approach from a patient viewpoint supports key trends in health-care – optimization of treatment results as well as improvement of overall experience for patients.
The economics of the market in pharmaceutical retinal biologics is linked to access and affordability. The expense associated with biological therapies can be high, causing doubts on available treatment options for patients as well as healthcare providers. This concern of the market is being addressed through attempts to generate biosimilars, develop cheap formulations and introduce patient support programs. The underlying motive behind these strategies is to make certain that improved retinal biologics are made available to a wider range of patients without reducing care quality.


















Leave a Comment