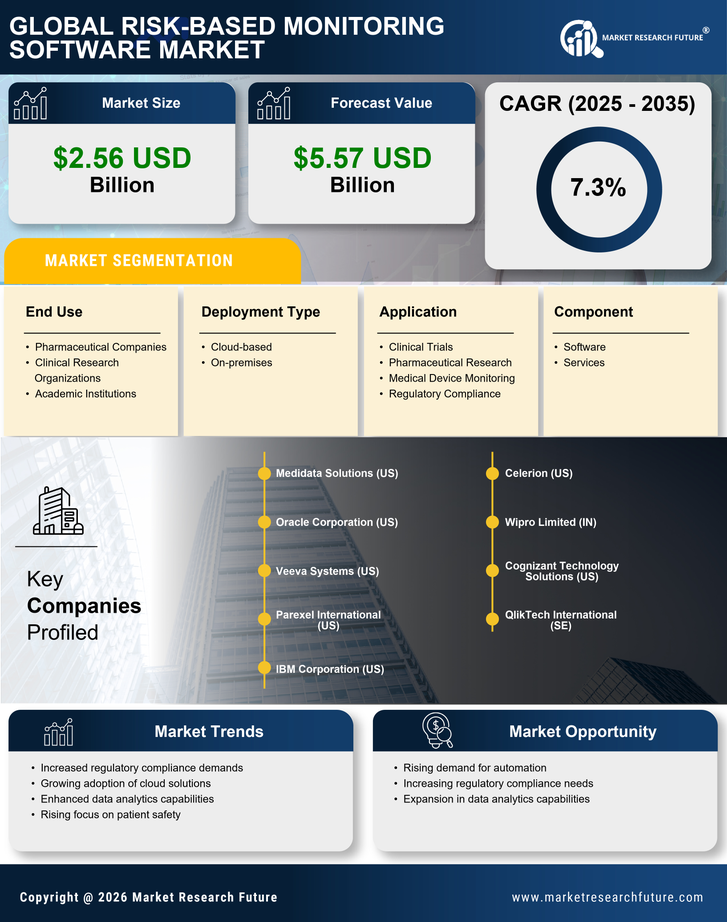
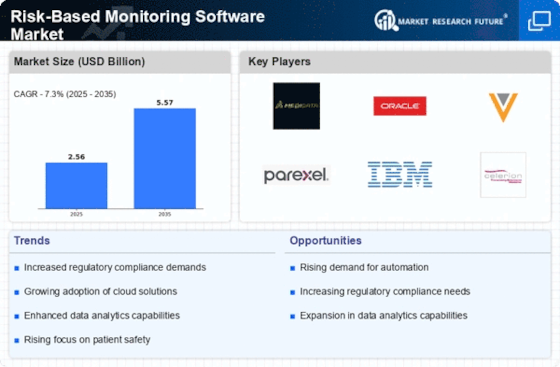
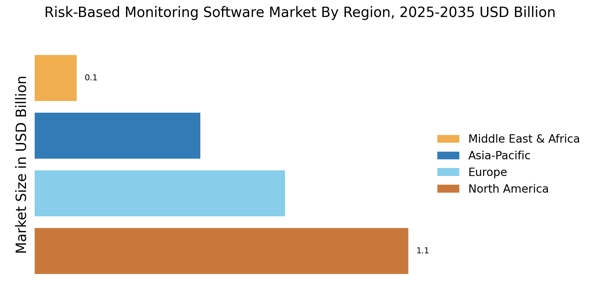
Integration of Advanced Analytics
The integration of advanced analytics into the Risk-Based Monitoring Software Market is transforming how organizations approach clinical trials. By leveraging data analytics, companies can identify potential risks and make informed decisions in real-time. This capability not only enhances the monitoring process but also contributes to better resource allocation and trial outcomes. The analytics segment within the software market is anticipated to grow at a compound annual growth rate of over 15% in the coming years. As organizations recognize the value of data-driven insights, the demand for risk-based monitoring solutions that incorporate advanced analytics is likely to increase, driving market growth.
Rising Focus on Patient-Centric Approaches
The Risk-Based Monitoring Software Market is witnessing a shift towards patient-centric approaches in clinical trials. This trend emphasizes the importance of patient engagement and experience, leading to the development of software solutions that prioritize these aspects. By utilizing risk-based monitoring, organizations can ensure that patient safety and well-being are at the forefront of their trial designs. This focus not only enhances patient retention but also improves overall trial efficiency. As the industry moves towards more patient-centered methodologies, the demand for risk-based monitoring software that aligns with these principles is expected to rise, fostering market expansion.
Regulatory Compliance and Quality Assurance
Regulatory compliance remains a critical driver for the Risk-Based Monitoring Software Market. With stringent regulations imposed by authorities such as the FDA and EMA, organizations are compelled to adopt software solutions that facilitate adherence to these guidelines. The software aids in maintaining quality assurance throughout the clinical trial process, thereby reducing the risk of non-compliance. As the industry evolves, the emphasis on data integrity and patient safety continues to grow, further propelling the demand for risk-based monitoring solutions. The market for compliance-related software is expected to expand significantly, reflecting the increasing importance of regulatory adherence in clinical research.
Growing Demand for Efficient Clinical Trials
The Risk-Based Monitoring Software Market is experiencing a surge in demand for efficient clinical trials. As pharmaceutical companies and clinical research organizations seek to optimize their trial processes, the need for software that can effectively monitor risks becomes paramount. This demand is driven by the increasing complexity of clinical trials, which often involve multiple sites and diverse patient populations. According to recent estimates, the clinical trial market is projected to reach USD 65 billion by 2027, indicating a robust growth trajectory. Consequently, the Risk-Based Monitoring Software Market is positioned to benefit from this trend, as organizations look for solutions that enhance trial efficiency while ensuring compliance with regulatory standards.
Increased Investment in Research and Development
Investment in research and development is a significant driver for the Risk-Based Monitoring Software Market. As organizations strive to innovate and improve their clinical trial processes, they are allocating substantial resources towards developing advanced monitoring solutions. This trend is reflected in the increasing number of partnerships and collaborations among technology providers and pharmaceutical companies. The R&D expenditure in the life sciences sector is projected to exceed USD 200 billion by 2026, indicating a strong commitment to enhancing trial methodologies. Consequently, the Risk-Based Monitoring Software Market stands to benefit from this influx of investment, as new technologies and solutions emerge to meet the evolving needs of clinical research.