Advancements in Biologic Therapies
Advancements in biologic therapies represent a critical driver for the Seropositive Rheumatoid Arthritis Drug Market. Biologics, which target specific components of the immune system, have transformed the treatment landscape for rheumatoid arthritis. The introduction of new biologic agents has provided patients with more effective options, leading to improved disease management and quality of life. Market data suggests that the biologic segment is expected to grow significantly, with a projected compound annual growth rate of over 8% in the coming years. This growth is fueled by ongoing clinical trials and the approval of novel therapies that demonstrate enhanced efficacy and safety profiles. As healthcare providers increasingly adopt these advanced treatments, the Seropositive Rheumatoid Arthritis Drug Market is likely to experience substantial growth, driven by the demand for innovative therapeutic solutions.
Increased Focus on Personalized Medicine
The increased focus on personalized medicine is shaping the Seropositive Rheumatoid Arthritis Drug Market. Personalized medicine aims to tailor treatments based on individual patient characteristics, including genetic profiles and disease biomarkers. This approach has gained traction in recent years, as it holds the potential to enhance treatment efficacy and minimize adverse effects. The market is witnessing a surge in the development of targeted therapies that align with this trend. For instance, the identification of specific biomarkers associated with seropositive rheumatoid arthritis has led to the creation of therapies that are more effective for certain patient subsets. As healthcare systems embrace personalized treatment paradigms, the Seropositive Rheumatoid Arthritis Drug Market is likely to expand, driven by the demand for customized therapeutic options that cater to the unique needs of patients.
Regulatory Support for New Drug Approvals
Regulatory support for new drug approvals is an influential driver for the Seropositive Rheumatoid Arthritis Drug Market. Regulatory agencies are increasingly streamlining the approval process for innovative therapies, recognizing the urgent need for effective treatments in the rheumatoid arthritis space. Initiatives aimed at expediting the review of new drugs, such as the FDA's Breakthrough Therapy designation, have encouraged pharmaceutical companies to pursue the development of novel agents. This supportive regulatory environment is likely to facilitate the entry of new therapies into the market, thereby enhancing treatment options for patients. As more drugs receive approval, the Seropositive Rheumatoid Arthritis Drug Market is expected to expand, driven by the availability of innovative therapies that address the diverse needs of the patient population.
Growing Investment in Research and Development
Growing investment in research and development is a pivotal driver for the Seropositive Rheumatoid Arthritis Drug Market. Pharmaceutical companies are increasingly allocating resources to discover and develop new therapies that address unmet medical needs in rheumatoid arthritis treatment. This trend is evidenced by the rising number of clinical trials focused on innovative drug candidates, including small molecules and biologics. Market analysis indicates that R&D spending in the rheumatology sector has seen a substantial increase, with many companies prioritizing the development of novel agents that target the underlying mechanisms of the disease. As these investments yield promising results, the Seropositive Rheumatoid Arthritis Drug Market is poised for growth, as new therapies enter the market and provide patients with more effective treatment options.
Rise in Prevalence of Seropositive Rheumatoid Arthritis
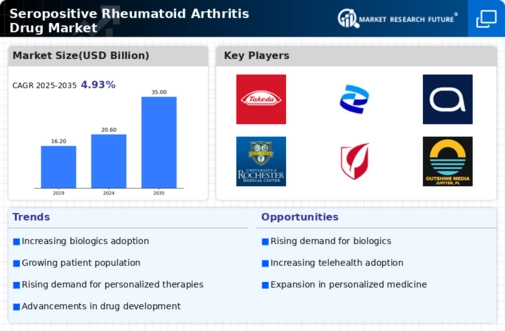
The increasing prevalence of seropositive rheumatoid arthritis is a notable driver for the Seropositive Rheumatoid Arthritis Drug Market. Recent estimates indicate that approximately 1.3 million individuals in the United States are affected by rheumatoid arthritis, with a significant proportion being seropositive. This growing patient population necessitates the development and availability of effective therapeutic options. As awareness of the disease rises, more patients seek treatment, thereby expanding the market. Furthermore, the aging population contributes to this trend, as the incidence of rheumatoid arthritis tends to increase with age. Consequently, pharmaceutical companies are likely to invest more in research and development to address the needs of this expanding demographic, which could lead to a broader range of treatment options in the Seropositive Rheumatoid Arthritis Drug Market.