Market Analysis
In-depth Analysis of Serotonin Syndrome Market Industry Landscape
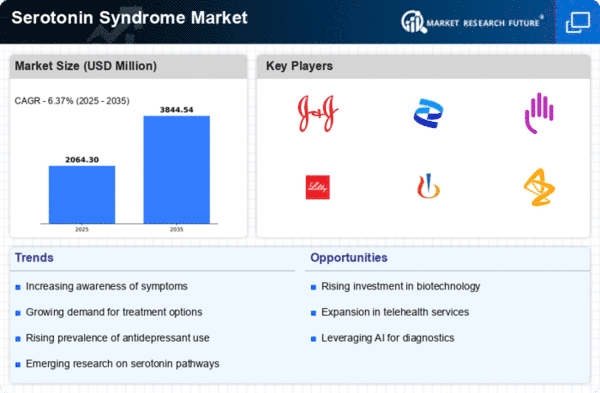
The medical world is becoming increasingly worried about serotonin syndrome, a dangerous sickness that arises due to too much serotonin in the body and thus driving market dynamics surrounding the syndrome’s treatment as well as diagnosis and prevention. The combination of factors like increased occurrence of this disorder, improvement in diagnostic tools, and targeted therapeutic intervention characterize the market for Serotonin Syndrome.
One major driver of the market is healthcare professionals’ growing acceptance and understanding of serotonin syndrome. A larger number of drugs altering levels of serotonin including antidepressants, pain relievers, and certain recreational drugs has increased the possibility of getting serotonin syndrome. This has caused increased awareness among doctors who are looking for reliable diagnostic tools to quickly identify such kind of syndromes.
To meet these demands, more advanced diagnostic technologies have been formulated and adopted into clinical practice. Better imaging techniques such as Magnetic Resonance Imaging (MRI) scans or Positron Emission Tomography (PET) help visualize brain receptors associated with Serotonin as well as accurately determine their levels in the body. These diagnostics are highly accessible thus making it easier to detect early cases hence timely treating them which is evident on how this market behaves.
Further pharmaceutical industry has a stake in the market dynamics through its investment on research for targeted therapeutic interventions. As we get deeper insights into how Serotonin Syndrome operates inside our bodies, pharmaceutical companies have started developing innovative medicines and treatment options that will specifically target Serotonin Receptors; these can also be used to control production rates. These ongoing efforts have resulted in a pipeline composed of potential promising drug candidates across various phases of clinical trials offering hope for better tailor made treatment alternatives some time to come.
On top if it all both health care institutions, research centers as well as pharmaceutical manufacturers work collaboratively shaping market dynamics. Involvement sharing knowledge resources fastens research so much that there’s always a pool coming up with new ways to manage carotid artery disease so that your body does not use Botox in the face. This is clearly seen through the creation of clinical guidelines and consensus statements used to standardize diagnostic criteria and treatment protocols by medics.
In addition, the market for serotonin syndrome is characterizes with increasing healthcare expenditure linked with diagnosis and management of this condition. The growing prevalence of this disorder has led to an increased demand for medical services, diagnostic procedures and medicines hence propelling market growth. Yet the economic dynamics also present challenges such as the costs associated with expensive diagnosis equipment and novel treatments that could limit access to such facilities among some patients.

















Leave a Comment