Rising Incidence of Influenza Cases
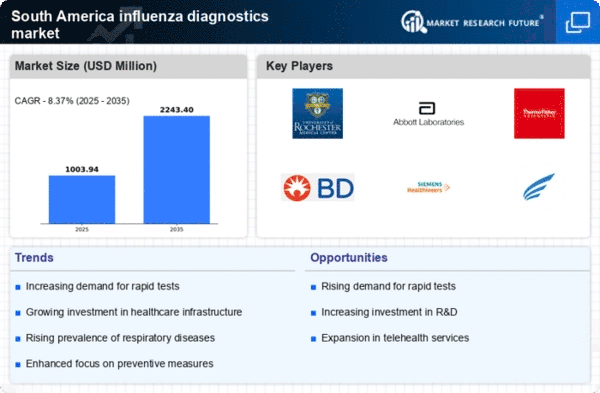
The increasing incidence of influenza cases in South America appears to be a primary driver for the influenza diagnostics market. Seasonal outbreaks and sporadic epidemics contribute to heightened awareness and demand for diagnostic testing. According to health authorities, the region has witnessed a rise in influenza-related hospitalizations, prompting healthcare providers to seek efficient diagnostic solutions. This trend indicates a potential growth in the market, as timely diagnosis is crucial for effective management and treatment. The influenza diagnostics market is likely to benefit from this rising incidence, as healthcare systems prioritize rapid and accurate testing to mitigate the impact of influenza outbreaks.
Expansion of Healthcare Infrastructure
The expansion of healthcare infrastructure in South America is a crucial driver for the influenza diagnostics market. Governments and private sectors are investing in healthcare facilities, particularly in rural and underserved areas. This expansion includes the establishment of diagnostic laboratories and clinics equipped with modern testing technologies. As access to healthcare improves, the demand for influenza diagnostics is expected to rise. The influenza diagnostics market stands to benefit from this trend, as enhanced infrastructure facilitates timely testing and treatment, ultimately leading to better management of influenza outbreaks.
Increased Public Awareness and Education
Increased public awareness and education regarding influenza prevention and treatment are driving the influenza diagnostics market in South America. Campaigns aimed at educating the population about the importance of vaccination and early diagnosis have led to a greater understanding of influenza's impact. This heightened awareness encourages individuals to seek diagnostic testing when symptoms arise, thereby increasing demand for influenza diagnostics. The influenza diagnostics market is likely to experience growth as more people recognize the value of early detection and treatment, contributing to better health outcomes and reduced transmission rates.
Regulatory Support for Diagnostic Innovations
Regulatory support for diagnostic innovations is emerging as a significant driver for the influenza diagnostics market in South America. Governments are increasingly recognizing the need for efficient diagnostic tools to combat infectious diseases. This has led to streamlined approval processes for new diagnostic tests, encouraging manufacturers to develop and introduce innovative solutions. The influenza diagnostics market is likely to thrive as regulatory bodies promote the adoption of advanced testing methods, ensuring that healthcare providers have access to the latest technologies for accurate and timely influenza diagnosis.
Technological Advancements in Diagnostic Tools
Technological advancements in diagnostic tools are significantly influencing the influenza diagnostics market in South America. Innovations such as rapid antigen tests and molecular assays enhance the speed and accuracy of influenza detection. The introduction of point-of-care testing devices allows for immediate results, which is essential in clinical settings. As healthcare facilities increasingly adopt these advanced technologies, the market is expected to expand. The influenza diagnostics market is likely to see a surge in demand for these innovative tools, as they facilitate timely interventions and improve patient outcomes, ultimately leading to a more efficient healthcare system.