Market Growth Projections
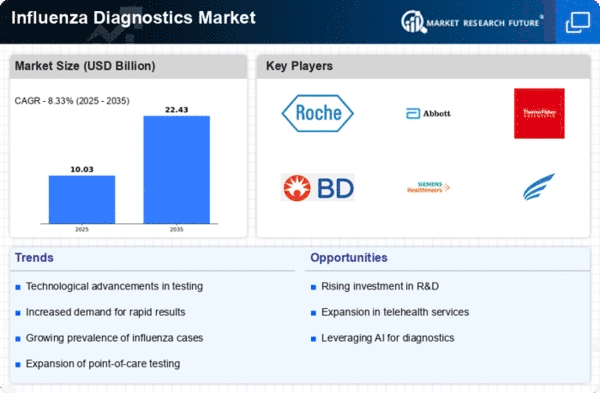
The Global Influenza Diagnostics Market Industry is poised for substantial growth, with projections indicating a market size of 7.22 USD Billion in 2024 and an anticipated increase to 11.9 USD Billion by 2035. This growth trajectory reflects a compound annual growth rate of 4.69% from 2025 to 2035. Such figures suggest a robust demand for influenza diagnostic solutions, driven by factors such as rising incidences of influenza, technological advancements, and increased healthcare access. The market's expansion is indicative of the critical role that effective diagnostics play in managing influenza outbreaks and protecting public health.
Rising Incidence of Influenza
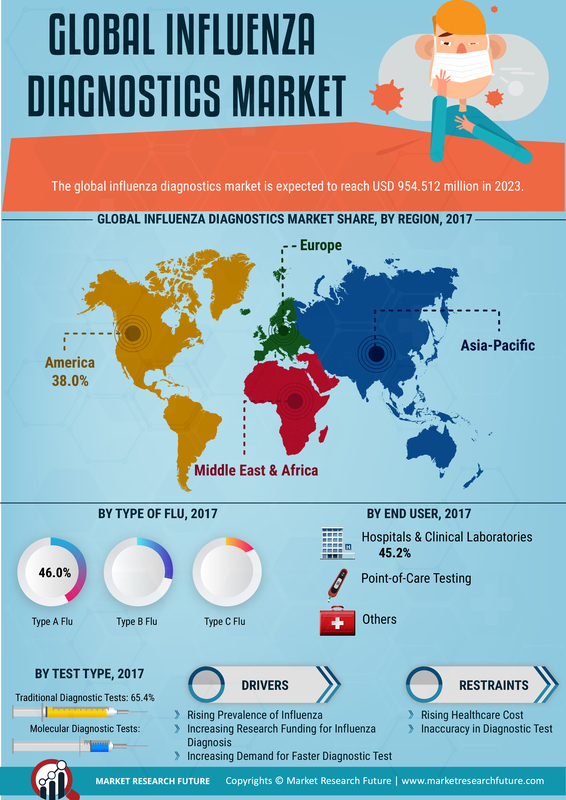
The Global Influenza Diagnostics Market Industry is experiencing growth due to the increasing incidence of influenza infections worldwide. Seasonal outbreaks and pandemics contribute to a heightened demand for diagnostic tools. For instance, the World Health Organization indicates that influenza affects millions of people annually, leading to significant morbidity and mortality. This rising incidence necessitates accurate and timely diagnostics, thereby driving the market. The market is projected to reach 7.22 USD Billion in 2024, reflecting the urgent need for effective influenza diagnostics to manage public health challenges.
Government Initiatives and Funding
Government initiatives aimed at improving public health infrastructure significantly influence the Global Influenza Diagnostics Market Industry. Increased funding for influenza surveillance and research programs enhances the development and distribution of diagnostic tools. For instance, various health departments worldwide allocate resources to combat influenza through vaccination campaigns and diagnostic improvements. These initiatives not only bolster the market but also ensure that healthcare systems are better equipped to handle influenza outbreaks. The anticipated compound annual growth rate of 4.69% from 2025 to 2035 underscores the positive impact of such government efforts on market growth.
Growing Awareness of Influenza Prevention
The Global Influenza Diagnostics Market Industry benefits from the growing awareness surrounding influenza prevention and control. Public health campaigns emphasize the importance of early diagnosis and treatment, leading to increased demand for diagnostic tests. Educational initiatives by health organizations aim to inform the public about the symptoms and risks associated with influenza, thereby promoting proactive healthcare behaviors. This heightened awareness contributes to a more informed population that seeks timely diagnostic solutions, further propelling market growth. As awareness continues to rise, the market is expected to expand in response to the demand for effective diagnostic tools.
Technological Advancements in Diagnostics
Technological innovations play a pivotal role in the Global Influenza Diagnostics Market Industry. The development of rapid diagnostic tests, including molecular assays and point-of-care testing, enhances the speed and accuracy of influenza detection. These advancements facilitate timely treatment and containment of outbreaks. For example, the introduction of multiplex PCR assays allows for the simultaneous detection of multiple respiratory pathogens, improving diagnostic efficiency. As technology continues to evolve, the market is likely to expand, with projections indicating a growth to 11.9 USD Billion by 2035, driven by the demand for advanced diagnostic solutions.
Emerging Markets and Increased Healthcare Access
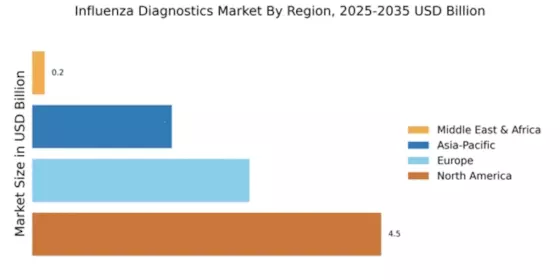
Emerging markets are becoming increasingly significant in the Global Influenza Diagnostics Market Industry. As healthcare access improves in developing regions, the demand for influenza diagnostics rises correspondingly. Countries in Asia and Africa are witnessing investments in healthcare infrastructure, leading to enhanced diagnostic capabilities. This trend is crucial as it allows for better disease management and surveillance, ultimately reducing the burden of influenza. The growth potential in these markets is substantial, with the overall market expected to grow steadily, driven by the increasing accessibility of diagnostic tools and services.