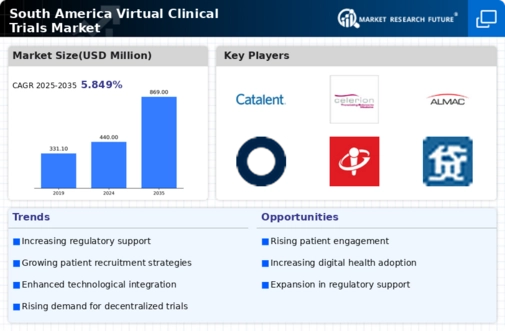
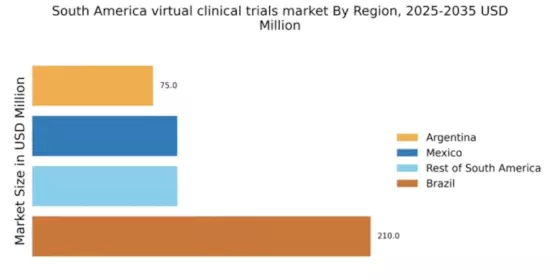
Cost Efficiency in Clinical Research
Cost efficiency is emerging as a significant driver for the virtual clinical-trials market. Traditional clinical trials often incur substantial expenses related to patient recruitment, site management, and logistics. In contrast, virtual trials can reduce these costs by leveraging digital platforms for patient engagement and data collection. Reports indicate that virtual trials can lower operational costs by up to 30%, making them an attractive option for sponsors and research organizations. This financial advantage is particularly relevant in South America, where budget constraints can limit the scope of clinical research. The virtual clinical-trials market is thus positioned to benefit from this cost-effective approach, enabling more studies to be conducted within limited budgets while maintaining high-quality standards.
Growing Focus on Real-World Evidence
The growing focus on real-world evidence (RWE) is significantly impacting the virtual clinical-trials market. As stakeholders in South America increasingly seek to understand the effectiveness of treatments in everyday settings, virtual trials provide a unique opportunity to gather RWE. By utilizing digital tools to collect data from diverse patient populations, researchers can gain insights that are more reflective of real-world scenarios. This trend is likely to enhance the credibility of clinical findings and support regulatory submissions. The virtual clinical-trials market is thus positioned to thrive as the demand for RWE continues to rise, enabling more informed decision-making in healthcare and potentially leading to improved patient outcomes.
Enhanced Patient Engagement and Retention
Enhanced patient engagement and retention are crucial factors driving the virtual clinical-trials market. The traditional model of clinical trials often faces challenges in maintaining participant interest and compliance. However, virtual trials offer innovative solutions, such as mobile applications and online platforms, that facilitate continuous communication and support. This approach appears to resonate well with patients, leading to improved retention rates. Studies suggest that virtual trials can achieve retention rates exceeding 80%, compared to 60% in conventional trials. This increased engagement is vital for the success of clinical research, as it ensures that data collected is robust and reliable. Consequently, the virtual clinical-trials market is likely to see continued growth as more organizations adopt these patient-centric strategies.
Regulatory Support for Digital Innovations
Regulatory support for digital innovations is a key driver influencing the virtual clinical-trials market. In South America, regulatory bodies are increasingly recognizing the potential of digital technologies to enhance clinical research. Initiatives aimed at streamlining the approval processes for virtual trials are being implemented, which may encourage more organizations to adopt these methodologies. The virtual clinical-trials market is likely to benefit from this supportive regulatory environment, as it fosters innovation and encourages investment in digital health solutions. Furthermore, as regulations evolve to accommodate new technologies, the potential for faster trial initiation and completion becomes more feasible, ultimately enhancing the overall efficiency of clinical research.
Increased Demand for Remote Healthcare Solutions
The rising demand for remote healthcare solutions is a pivotal driver in the virtual clinical-trials market. As healthcare systems in South America evolve, there is a noticeable shift towards telemedicine and remote patient monitoring. This trend is likely fueled by the need for accessible healthcare, particularly in rural areas where traditional clinical trial participation may be limited. The virtual clinical-trials market is experiencing a surge, with estimates suggesting a growth rate of approximately 15% annually. This growth is indicative of a broader acceptance of digital health solutions, which are becoming integral to clinical research methodologies. The convenience and efficiency offered by virtual trials are appealing to both patients and researchers, potentially leading to higher enrollment rates and improved data collection processes.