Advancements in Biotechnology
Technological advancements in biotechnology are significantly impacting the orphan diseases market. Innovations such as gene therapy, CRISPR technology, and personalized medicine are paving the way for novel treatment options. These advancements enable researchers to target the underlying genetic causes of rare diseases, which were previously deemed untreatable. The orphan diseases market is likely to benefit from these breakthroughs, as they can lead to the development of more effective therapies. In 2025, the market for gene therapies alone is expected to exceed $10 billion, highlighting the potential for growth driven by biotechnological innovations. As these technologies continue to evolve, they may reshape the landscape of the orphan diseases market.
Regulatory Framework Enhancements
The regulatory environment surrounding the orphan diseases market is evolving, with enhancements aimed at facilitating drug development. The US Food and Drug Administration (FDA) has implemented various initiatives to streamline the approval process for orphan drugs, including the Orphan Drug Designation program. This program provides incentives such as tax credits and market exclusivity, encouraging pharmaceutical companies to invest in rare disease therapies. As a result, the orphan diseases market is likely to see an increase in the number of approved treatments, which could enhance patient access to necessary therapies. The ongoing regulatory support may further stimulate growth in the orphan diseases market, fostering a more favorable environment for innovation.
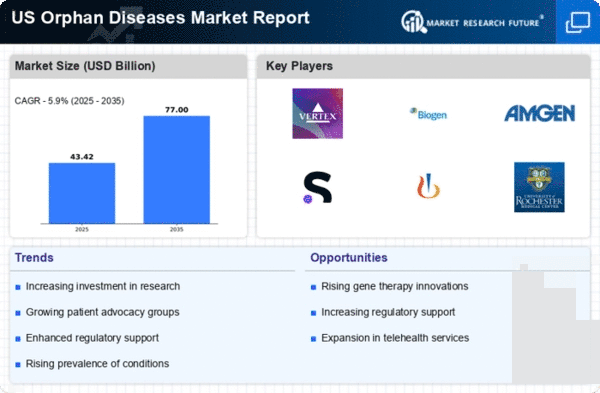
Rising Prevalence of Orphan Diseases
The orphan diseases market is experiencing growth due to the increasing prevalence of rare diseases in the US. According to the National Institutes of Health, approximately 7,000 rare diseases affect an estimated 30 million Americans. This rising number of patients creates a pressing need for effective treatments, thereby driving investment in the orphan diseases market. Pharmaceutical companies are increasingly focusing on developing therapies for these conditions, as the potential for high returns on investment becomes evident. The orphan diseases market is projected to reach $200 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 10%. This trend indicates a robust demand for innovative solutions tailored to the unique challenges posed by rare diseases.
Growing Investment from Venture Capital
Venture capital investment in the orphan diseases market is on the rise, reflecting a growing interest in rare disease therapies. Investors are increasingly recognizing the potential for high returns in this niche market, as successful treatments can command premium pricing due to limited competition. In 2025, venture capital funding for orphan drug development is projected to reach $5 billion, indicating a robust financial commitment to addressing unmet medical needs. This influx of capital is likely to stimulate innovation and accelerate the development of new therapies, further propelling the growth of the orphan diseases market. As more investors enter this space, the landscape for rare disease treatments may become increasingly dynamic.
Increased Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, academic institutions, and patient advocacy groups, is becoming increasingly prevalent in the orphan diseases market. This trend fosters a more integrated approach to research and development, allowing for the sharing of resources and expertise. Such partnerships can accelerate the development of new therapies and improve patient access to treatments. For instance, initiatives like the Orphan Drug Act encourage collaboration by providing incentives for companies to work together on orphan drug development. This collaborative environment is expected to enhance the orphan diseases market, potentially leading to a more efficient drug development process and improved patient outcomes.
.png)