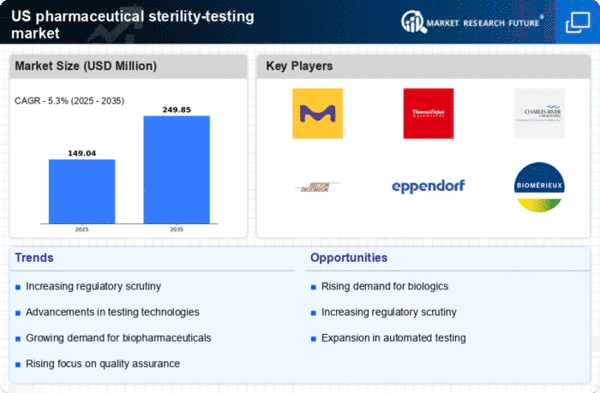
The pharmaceutical sterility-testing market is characterized by a competitive landscape that is increasingly shaped by innovation, regulatory compliance, and the demand for high-quality products. Key players such as Thermo Fisher Scientific Inc. (US), Charles River Laboratories International Inc. (US), and Merck KGaA (DE) are at the forefront, employing strategies that emphasize technological advancements and strategic partnerships. These companies are not only focusing on enhancing their product offerings but are also investing in digital transformation initiatives to streamline operations and improve customer engagement, thereby collectively shaping a dynamic competitive environment. In terms of business tactics, companies are increasingly localizing manufacturing to reduce lead times and optimize supply chains. The market structure appears moderately fragmented, with several players vying for market share. However, the influence of major companies is significant, as they leverage their resources to establish a competitive edge through innovation and operational efficiency. In October 2025, Thermo Fisher Scientific Inc. (US) announced the launch of a new line of sterility testing products designed to enhance the accuracy and speed of microbial detection. This strategic move is likely to bolster their market position by addressing the growing demand for rapid testing solutions, which is critical in maintaining compliance with stringent regulatory standards. The introduction of these products may also facilitate the expansion of their customer base, particularly among biopharmaceutical manufacturers. In September 2025, Charles River Laboratories International Inc. (US) expanded its service offerings by acquiring a leading sterility testing laboratory. This acquisition is expected to enhance their capabilities in providing comprehensive testing solutions, thereby strengthening their competitive position. The integration of this laboratory into their existing operations may allow for improved service delivery and increased market penetration, particularly in the biopharmaceutical sector. In August 2025, Merck KGaA (DE) entered into a strategic partnership with a technology firm to develop AI-driven sterility testing solutions. This collaboration is indicative of a broader trend towards the integration of advanced technologies in testing processes. By leveraging AI, Merck KGaA aims to enhance the efficiency and accuracy of sterility testing, which could set new industry standards and provide a competitive advantage in a rapidly evolving market. As of November 2025, current trends in the pharmaceutical sterility-testing market include a pronounced shift towards digitalization, sustainability, and the integration of AI technologies. Strategic alliances are increasingly shaping the competitive landscape, enabling companies to pool resources and expertise. Looking ahead, it appears that competitive differentiation will evolve from traditional price-based competition to a focus on innovation, technological advancements, and supply chain reliability, suggesting a transformative period for the industry.
.png)