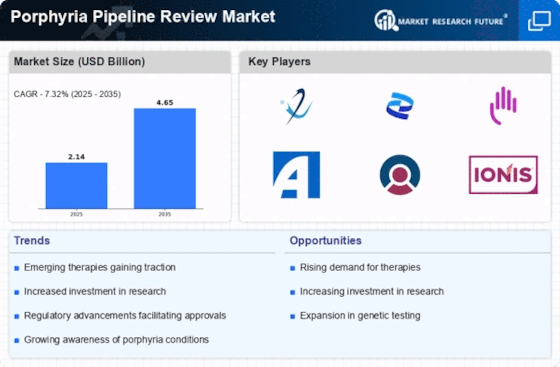
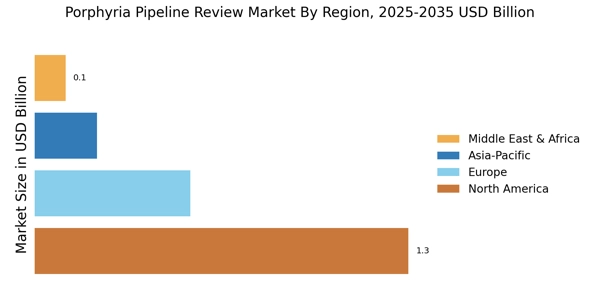
The Porphyria Pipeline Review Market is characterized by a dynamic competitive landscape, driven by the increasing prevalence of porphyria disorders and the growing demand for innovative therapies. Key players such as Alnylam Pharmaceuticals (US), Pfizer (US), and Horizon Therapeutics (IE) are strategically positioned to leverage their research capabilities and extensive portfolios. Alnylam Pharmaceuticals (US) focuses on RNA interference technology, which appears to be a promising avenue for treating acute porphyrias. Meanwhile, Pfizer (US) is enhancing its presence through strategic partnerships aimed at expanding its therapeutic offerings in rare diseases. Horizon Therapeutics (IE) emphasizes its commitment to patient-centric solutions, which may enhance its competitive edge in the market. Collectively, these strategies suggest a trend towards innovation and collaboration, shaping a competitive environment that prioritizes advanced therapeutic solutions.
In terms of business tactics, companies are increasingly localizing manufacturing and optimizing supply chains to enhance efficiency and responsiveness to market demands. The Porphyria Pipeline Review Market is moderately fragmented, with several players vying for market share. However, the collective influence of major companies like Alnylam Pharmaceuticals (US) and Pfizer (US) indicates a trend towards consolidation, as these firms seek to strengthen their market positions through strategic alliances and acquisitions.
In August 2025, Alnylam Pharmaceuticals (US) announced a collaboration with a leading biotechnology firm to develop a novel RNAi therapeutic specifically targeting acute intermittent porphyria. This partnership is significant as it not only expands Alnylam's pipeline but also underscores the potential of RNA interference technology in addressing unmet medical needs in porphyria treatment. The collaboration may enhance Alnylam's competitive positioning by accelerating the development timeline and broadening its therapeutic reach.
In September 2025, Pfizer (US) launched a new clinical trial for its investigational therapy aimed at treating hereditary coproporphyria. This strategic move reflects Pfizer's commitment to advancing its portfolio in rare diseases and demonstrates its proactive approach to addressing the needs of patients suffering from porphyria. The trial's outcomes could potentially position Pfizer as a leader in this niche market, further solidifying its reputation for innovation in therapeutic development.
In July 2025, Horizon Therapeutics (IE) expanded its global footprint by entering into a strategic partnership with a European pharmaceutical company to enhance access to its therapies for porphyria patients. This expansion is indicative of Horizon's strategy to increase its market presence and improve patient access to essential treatments. Such partnerships may facilitate the sharing of resources and expertise, ultimately benefiting patients and enhancing Horizon's competitive stance in the market.
As of October 2025, the Porphyria Pipeline Review Market is witnessing trends that emphasize digitalization, sustainability, and the integration of artificial intelligence in drug development. Strategic alliances are increasingly shaping the competitive landscape, allowing companies to pool resources and expertise to accelerate innovation. Looking ahead, it appears that competitive differentiation will evolve from traditional price-based competition to a focus on technological advancements, innovative therapies, and reliable supply chains, thereby enhancing patient outcomes and fostering long-term growth in the market.